Korean Circ J.
2017 Sep;47(5):663-669. 10.4070/kcj.2017.0028.
Novel Concept of a Heart-Gut Axis in the Pathophysiology of Heart Failure
- Affiliations
-
- 1Department of Cardiovascular Medicine, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan. akazawah-tky@umin.ac.jp, komuro-tky@umin.ac.jp
- 2Department of Advanced Clinical Science and Therapeutics, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan.
- KMID: 2389597
- DOI: http://doi.org/10.4070/kcj.2017.0028
Abstract
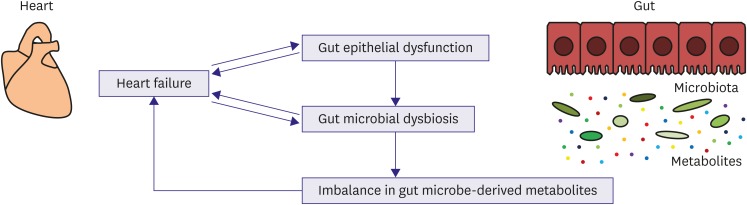
- Patients with heart failure (HF) have structural and functional changes of the gut as a result of microcirculatory disturbances. A disrupted gut epithelial barrier may lead to translocation of microbial products into systemic circulation, possibly aggravating HF by inducing inflammatory responses. Gut microbiota play an essential role in the maintenance of host homeostasis because large quantities of their gene products complement host physiological processes. Emerging evidence has suggested the potential clinical significance of gut microbiota in the pathophysiology of HF. Imbalances of gut microbe-derived metabolites can contribute to cardiac dysfunction and other morbidities in patients with HF. Therapeutic research for HF through targeting microbiota is under way. Thus, the novel concept of a heart-gut axis may lead to breakthroughs in the development of innovative diagnostics and therapeutic approaches for HF.
MeSH Terms
Figure
Reference
-
1. Verbrugge FH, Dupont M, Steels P, et al. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol. 2013; 62:485–495. PMID: 23747781.2. Rogler G, Rosano G. The heart and the gut. Eur Heart J. 2014; 35:426–430. PMID: 23864132.3. Nagatomo Y, Tang WH. Intersections between microbiome and heart failure: revisiting the gut hypothesis. J Card Fail. 2015; 21:973–980. PMID: 26435097.4. Sundaram V, Fang JC. Gastrointestinal and liver issues in heart failure. Circulation. 2016; 133:1696–1703. PMID: 27143152.5. Kamo T, Akazawa H, Suda W, et al. Dysbiosis and compositional alterations with aging in the gut microbiota of patients with heart failure. PLoS One. 2017; 12:e0174099. PMID: 28328981.6. Sandek A, Swidsinski A, Schroedl W, et al. Intestinal blood flow in patients with chronic heart failure: a link with bacterial growth, gastrointestinal symptoms, and cachexia. J Am Coll Cardiol. 2014; 64:1092–1102. PMID: 25212642.7. Sandek A, Bauditz J, Swidsinski A, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007; 50:1561–1569. PMID: 17936155.8. Arutyunov GP, Kostyukevich OI, Serov RA, Rylova NV, Bylova NA. Collagen accumulation and dysfunctional mucosal barrier of the small intestine in patients with chronic heart failure. Int J Cardiol. 2008; 125:240–245. PMID: 18242735.9. Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990; 323:236–241. PMID: 2195340.10. Milani RV, Mehra MR, Endres S, et al. The clinical relevance of circulating tumor necrosis factor-α in acute decompensated chronic heart failure without cachexia. Chest. 1996; 110:992–995. PMID: 8874257.11. Rauchhaus M, Doehner W, Francis DP, et al. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. 2000; 102:3060–3067. PMID: 11120695.12. Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation. 2001; 103:2055–2059. PMID: 11319194.13. Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT; Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-α, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003; 107:3133–3140. PMID: 12796126.14. Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004; 109:1594–1602. PMID: 15023878.15. Niebauer J, Volk HD, Kemp M, et al. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet. 1999; 353:1838–1842. PMID: 10359409.16. Peschel T, Schönauer M, Thiele H, Anker SD, Schuler G, Niebauer J. Invasive assessment of bacterial endotoxin and inflammatory cytokines in patients with acute heart failure. Eur J Heart Fail. 2003; 5:609–614. PMID: 14607199.17. Li J, Jia H, Cai X, et al. An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol. 2014; 32:834–841. PMID: 24997786.18. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014; 157:121–141. PMID: 24679531.19. Fischbach MA, Segre JA. Signaling in host-associated microbial communities. Cell. 2016; 164:1288–1300. PMID: 26967294.20. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016; 375:2369–2379. PMID: 27974040.21. Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016; 65:330–339. PMID: 26338727.22. Wang F, Li Q, Wang C, Tang C, Li J. Dynamic alteration of the colonic microbiota in intestinal ischemia-reperfusion injury. PLoS One. 2012; 7:e42027. PMID: 22848694.23. Phillips Campbell RB, Duffourc MM, Schoborg RV, et al. Aberrant fecal flora observed in guinea pigs with pressure overload is mitigated in animals receiving vagus nerve stimulation therapy. Am J Physiol Gastrointest Liver Physiol. 2016; 311:G754–G762. PMID: 27562060.24. Pasini E, Aquilani R, Testa C, et al. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail. 2016; 4:220–227. PMID: 26682791.25. Wikoff WR, Anfora AT, Liu J, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. 2009; 106:3698–3703. PMID: 19234110.26. Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016; 22:1079–1089. PMID: 27711063.27. Lekawanvijit S. Role of gut-derived protein-bound uremic toxins in cardiorenal syndrome and potential treatment modalities. Circ J. 2015; 79:2088–2097. PMID: 26346172.28. Yang K, Wang C, Nie L, et al. Klotho protects against indoxyl sulphate-induced myocardial hypertrophy. J Am Soc Nephrol. 2015; 26:2434–2446. PMID: 25804281.29. Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011; 472:57–63. PMID: 21475195.30. Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013; 368:1575–1584. PMID: 23614584.31. Tang WH, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest. 2014; 124:4204–4211. PMID: 25271725.32. Tang WH, Wang Z, Fan Y, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014; 64:1908–1914. PMID: 25444145.33. Tang WH, Wang Z, Shrestha K, et al. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail. 2015; 21:91–96. PMID: 25459686.34. Trøseid M, Ueland T, Hov JR, et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med. 2015; 277:717–726. PMID: 25382824.35. Suzuki T, Heaney LM, Bhandari SS, Jones DJ, Ng LL. Trimethylamine N-oxide and prognosis in acute heart failure. Heart. 2016; 102:841–848. PMID: 26869641.36. Organ CL, Otsuka H, Bhushan S, et al. Choline diet and its gut microbe-derived metabolite, trimethylamine N-oxide, exacerbate pressure overload-induced heart failure. Circ Heart Fail. 2016; 9:e002314. PMID: 26699388.37. van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile . N Engl J Med. 2013; 368:407–415. PMID: 23323867.38. Wang Z, Roberts AB, Buffa JA, et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015; 163:1585–1595. PMID: 26687352.39. Karbach SH, Schönfelder T, Brandão I, et al. Gut microbiota promote angiotensin II-induced arterial hypertension and vascular dysfunction. J Am Heart Assoc. 2016; 5:e003698. PMID: 27577581.40. Lam V, Su J, Koprowski S, et al. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J. 2012; 26:1727–1735. PMID: 22247331.41. Gan XT, Ettinger G, Huang CX, et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ Heart Fail. 2014; 7:491–499. PMID: 24625365.42. Marques FZ, Nelson EM, Chu PY, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017; 135:964–977. PMID: 27927713.43. Lekawanvijit S, Kompa AR, Manabe M, et al. Chronic kidney disease-induced cardiac fibrosis is ameliorated by reducing circulating levels of a non-dialysable uremic toxin, indoxyl sulfate. PLoS One. 2012; 7:e41281. PMID: 22829936.44. Costanza AC, Moscavitch SD, Faria Neto HC, Mesquita ET. Probiotic therapy with Saccharomyces boulardii for heart failure patients: a randomized, double-blind, placebo-controlled pilot trial. Int J Cardiol. 2015; 179:348–350. PMID: 25464484.45. Zmora N, Zeevi D, Korem T, Segal E, Elinav E. Taking it personally: personalized utilization of the human microbiome in health and disease. Cell Host Microbe. 2016; 19:12–20. PMID: 26764593.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Arterial-Cardiac Interaction: The Concept and Implications
- Role of Beta-blockers in Treatment of Heart Failure
- Pathophysiology of Pediatric Heart Failure
- The Pathophysiology and Diagnostic Approaches for Diastolic Left Ventricular Dysfunction: A Clinical Perspective
- Key Role of the Korean Society of Heart Failure: Moving Towards a Global and Individualized Approach