Ann Rehabil Med.
2017 Apr;41(2):171-177. 10.5535/arm.2017.41.2.171.
Resting-State Metabolism of Hand Knob Area on ¹â¸F-FDG PET-CT According to Hand Function and Tractography of Corticospinal Tract After Stroke
- Affiliations
-
- 1Department of Nuclear Medicine, Keimyung University School of Medicine, Daegu, Korea.
- 2Department of Physical Medicine and Rehabilitation, Yeungnam University College of Medicine, Daegu, Korea. hikkali@hanmail.net
- KMID: 2389473
- DOI: http://doi.org/10.5535/arm.2017.41.2.171
Abstract
OBJECTIVE
To correlate the resting metabolism of hand knob and hand function after stroke, diffuse tensor tractography (DTT) and ¹â¸F-fluorodeoxyglucose position emission tomography (¹â¸F-FDG PET) were used to evaluate constructible state of white matter tract and metabolic state of gray matter, respectively.
METHODS
A total of 17 patients were included in the study, who had suffered a stroke with hand weakness, after a stroke. They underwent diffusion tensor analysis and FDG PET in the subacute period. The ratio of both hemisphere parameters in voxel number of fibers, fractional anisotropy (FA) and apparent diffusion coefficient obtained by corticospinal tract as constructed by DTT, and the metabolism of hand knob area on cerebral cortex obtained from ¹â¸F-FDG PET were calculated. Hand movement scale was evaluated on the day of FDG PET or tractography, and at 6 months after onset.
RESULTS
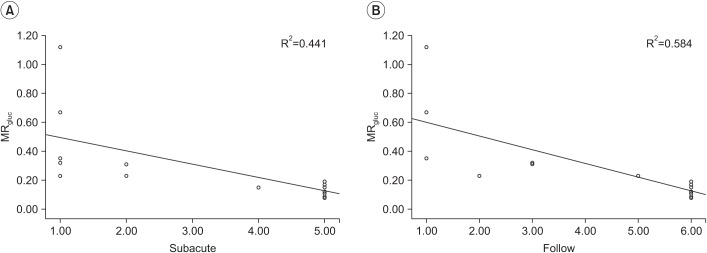
Difference of FA in DTT between both hemispheres and hand knob metabolism in FDG PET significantly correlated with the hand movement scale at the subacute stage and 6 months after onset. However, the difference of both hemispheres in DTT and metabolism of hand knob area was not significant.
CONCLUSION
Resting metabolism on hand knob in FDG PET correlated with hand function after stroke.
MeSH Terms
Figure
Reference
-
1. Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009; 8:741–754. PMID: 19608100.
Article2. Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999; 45:265–269. PMID: 9989633.
Article3. Cho SH, Kim DG, Kim DS, Kim YH, Lee CH, Jang SH. Motor outcome according to the integrity of the corticospinal tract determined by diffusion tensor tractography in the early stage of corona radiata infarct. Neurosci Lett. 2007; 426:123–127. PMID: 17897782.
Article4. Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst. 2002; 18:386–404. PMID: 12192499.
Article5. Dimitrakopoulou-Strauss A, Strauss LG, Burger C. Quantitative PET studies in pretreated melanoma patients: a comparison of 6-[18F]fluoro-L-dopa with 18F-FDG and (15)O-water using compartment and noncompartment analysis. J Nucl Med. 2001; 42:248–256. PMID: 11216523.6. Katrak P, Bowring G, Conroy P, Chilvers M, Poulos R, McNeil D. Predicting upper limb recovery after stroke: the place of early shoulder and hand movement. Arch Phys Med Rehabil. 1998; 79:758–761. PMID: 9685087.
Article7. Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991; 1:1–47. PMID: 1822724.
Article8. Jang SH, Cho SH, Kim YH, Kwon YH, Byun WM, Lee SJ, et al. Cortical activation changes associated with motor recovery in patients with precentral knob infarct. Neuroreport. 2004; 15:395–399. PMID: 15094490.
Article9. Seghier ML. Laterality index in functional MRI: methodological issues. Magn Reson Imaging. 2008; 26:594–601. PMID: 18158224.
Article10. Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, et al. Localization of the motor hand area to a knob on the precentral gyrus: a new landmark. Brain. 1997; 120:141–157. PMID: 9055804.
Article11. Dukart J, Mueller K, Horstmann A, Vogt B, Frisch S, Barthel H, et al. Differential effects of global and cerebellar normalization on detection and differentiation of dementia in FDG-PET studies. Neuroimage. 2010; 49:1490–1495. PMID: 19770055.
Article12. Kushner M, Tobin M, Alavi A, Chawluk J, Rosen M, Fazekas F, et al. Cerebellar glucose consumption in normal and pathologic states using fluorine-FDG and PET. J Nucl Med. 1987; 28:1667–1670. PMID: 3499490.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Corticospinal Tract Compression by Hematoma in a Patient with Intracerebral Hemorrhage: A Diffusion Tensor Tractography and Functional MRI Study
- ¹â¸F-FDG PET/MR Refines Evaluation in Newly Diagnosed Metastatic Urethral Adenocarcinoma
- Comparison of Neck CT and ¹â¸F-FDG PET-CT for Making the Preoperative Diagnosis of Lymph Node Metastasis in Papillary Thyroid Cancer
- â¶â¸Gallium-Arginine-Glycine-Aspartic Acid and ¹â¸F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Chondroblastic Osteosarcoma of the Skull
- Role of ¹â¸F-FDG PET-CT in Monitoring the Cyclophosphamide Induced Pulmonary Toxicity in Patients with Breast Cancer: 2 Case Reports