Yonsei Med J.
2006 Feb;47(1):135-139. 10.3349/ymj.2006.47.1.135.
Corticospinal Tract Compression by Hematoma in a Patient with Intracerebral Hemorrhage: A Diffusion Tensor Tractography and Functional MRI Study
- Affiliations
-
- 1Department of Physical Medicine and Rehabilitation, School of Medicine, Yeungnam University Daegu, Korea. kwonpt@lycos.co.kr
- 2Department of Rehabilitation Science, Graduate School, Daegu University, Daegu, Korea.
- 3Department of Rehabilitation Technology, Korea Nasarene University, Chonan, Korea.
- 4Department of Neurosurgery, School of Medicine, Yeungnam University, Daegu, Korea.
- KMID: 1715884
- DOI: http://doi.org/10.3349/ymj.2006.47.1.135
Abstract
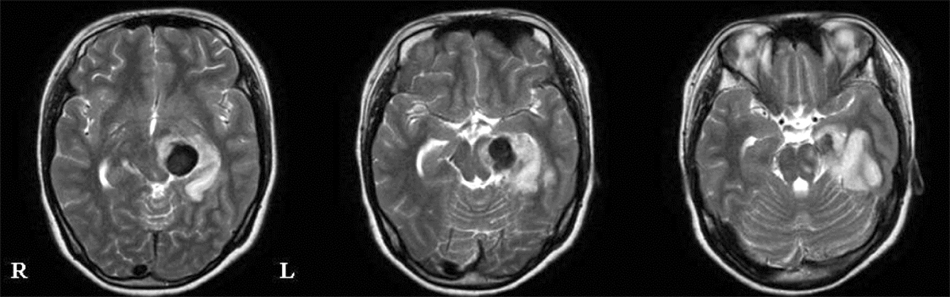
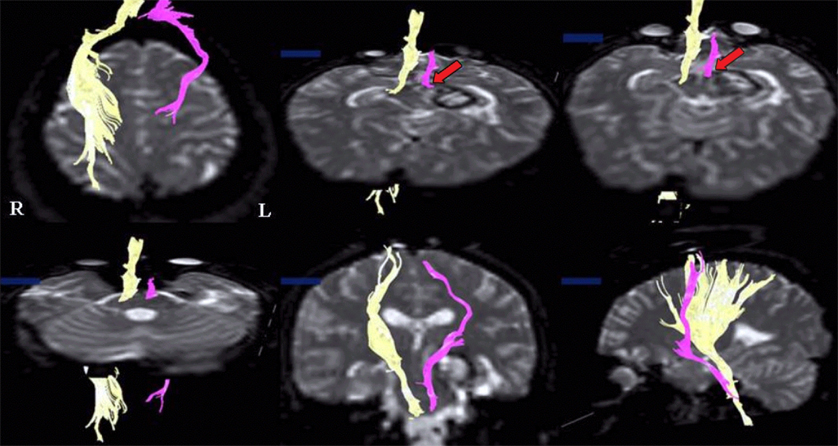
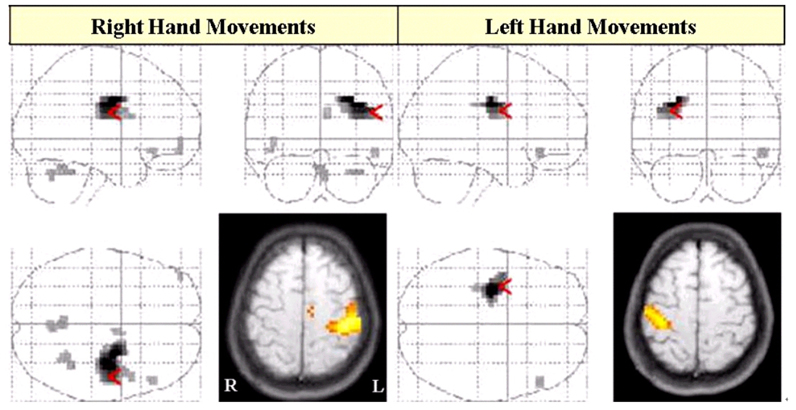
- The purpose of this study was to demonstrate corticospinal tract compression that was due to a hematoma by using diffusion tensor tractography (DTT) and functional MRI (fMRI) in a patient with an intracerebral hemorrhage (ICH). A 23-year-old right-handed woman presented with severe paralysis of her right extremities at the onset of a spontaneous ICH. Over the first three days from onset, the motor function of the affected upper and lower extremities rapidly recovered to the extent that she was able to overcome applied resistance to the affected limbs, and her limbs regained normal function 3 weeks after onset. The tract of the right hemisphere originated from the primary sensori-motor cortex (SM1) and it passed through the known corticospinal tract pathway. However, the tract of the left hemisphere was similar to that of the right hemisphere except that it was displaced to the antero-medial side by the hematoma at the cerebral peduncle. Only the contralateral SM1 area centered on the precentral knob was activated during affected (right) or unaffected (left) hand movements, respectively. In conclusion, fMRI and DTT demonstrated a corticospinal tract compression due to hematoma in this patient. We conclude that the combined use of these two modalities appears to improve the accuracy of investigating the state of the corticospinal tract.
MeSH Terms
Figure
Reference
-
1. Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997. 28:2518–2527.2. Jang SH, Cho SH, Kim YH, Kwon YH, Byun WM, Lee SJ, et al. Cortical activation changes associated with motor recovery in patients with precentral knob infarct. Neuroreport. 2004. 15:395–399.3. Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults: a review. Stroke. 2003. 34:1553–1566.4. Mori S, van Zijl PC. Fiber tracking: principles and strategies - a technical review. NMR Biomed. 2002. 15:468–480.5. Yamada K, Mori S, Nakamura H, Ito H, Kizu O, Shiga K, et al. Fiber-tracking method reveals sensorimotor pathway involvement in stroke patients. Stroke. 2003. 34:E159–E162.6. Lee SK, Mori S, Kim DJ, Kim SY, Kim SY, Chu M, et al. Diffusion tensor MRI and fiber tractography of cerebellar atrophy in phenytoin users. Epilepsia. 2003. 44:1536–1540.7. Lee SK, Mori S, Kim DJ, Kim SY, Kim SY, Kim DI. Diffusion tensor MR imaging visualizes the altered hemispheric fiber connection in callosal dysgenesis. Am J Neuroradiol. 2004. 25:25–28.8. Kim YH, Jang SH, Han BS, Kwon YH, You SH, Byun WM, et al. Ipsilateral motor pathway confirmed by diffusion tensor tractography in a patient with schizencephaly. Neuroreport. 2004. 15:1899–1902.9. Macdonell RA, Jackson GD, Curatolo JM, Abbott DF, Berkovic SF, Carey LM, et al. Motor cortex localization using functional MRI and transcranial magnetic stimulation. Neurology. 1999. 53:1462–1467.10. Kim YT, Kang SY, Kim HS, Shin BS. Hand strength and dextricity evaluation with age. J Korean Acad Rehab. 1994. 18:780–788.11. Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999. 45:265–269.12. Witte OW. Lesion-induced plasticity as a potential mechanism for recovery and rehabilitative training. Curr Opin Neurol. 1998. 11:655–662.13. Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997. 120:141–157.14. Bastings EP, Rapisarda G, Pennisi G, Noordhout AM, Lenaerts M, Good DC, et al. Mechanisms of hand motor recovery after stroke: an electrophysiologic study of central motor pathways. J Neuro Rehabil. 1997. 11:97–108.15. Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci. 2001. 21:5272–5280.16. Binkofski F, Seitz RJ, Arnold S, Classen J, Benecke R, Freund HJ. Thalamic metabolism and corticospinal tract integrity determine motor recovery in stroke. Ann Neurol. 1997. 39:460–470.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of Corticospinal Tract Injury by Using the Diffusion Tensor Imaging of 3.0 T Magnetic Resonance in Patients with Hypertensive Intracerebral Hemorrhage
- Applicability of Diffusion Tensor MR Imaging and Fiber Tractography That Predict Short-Term Functional Motor Outcome in Patients with Deep Intracerebral Hemorrhage
- Clinical Usefulness of Diffusion Tensor Image Tractography in Stroke Patients: Report of two cases
- Diffusion Tensor Tractography in Two Cases of Kernohan-Woltman Notch Phenomenon
- Mini-Review of Studies Reporting the Repeatability and Reproducibility of Diffusion Tensor Imaging