Korean J Physiol Pharmacol.
2016 Mar;20(2):221-228. 10.4196/kjpp.2016.20.2.221.
Effects of prunetin on the proteolytic activity, secretion and gene expression of MMP-3 in vitro and production of MMP-3 in vivo
- Affiliations
-
- 1Department of Orthopedic Surgery and Institute of Health Sciences, School of Medicine and Hospital, Gyeongsang National University, Jinju 52727, Korea. hscspine@hanmail.net LCJ123@cnu.ac.kr
- 2Department of Orthopedic Surgery, School of Medicine, Chungnam National University, Daejeon 35015, Korea.
- 3Department of Pharmacology, School of Medicine, Chungnam National University, Daejeon 35015, Korea. hscspine@hanmail.net LCJ123@cnu.ac.kr
- KMID: 2388679
- DOI: http://doi.org/10.4196/kjpp.2016.20.2.221
Abstract
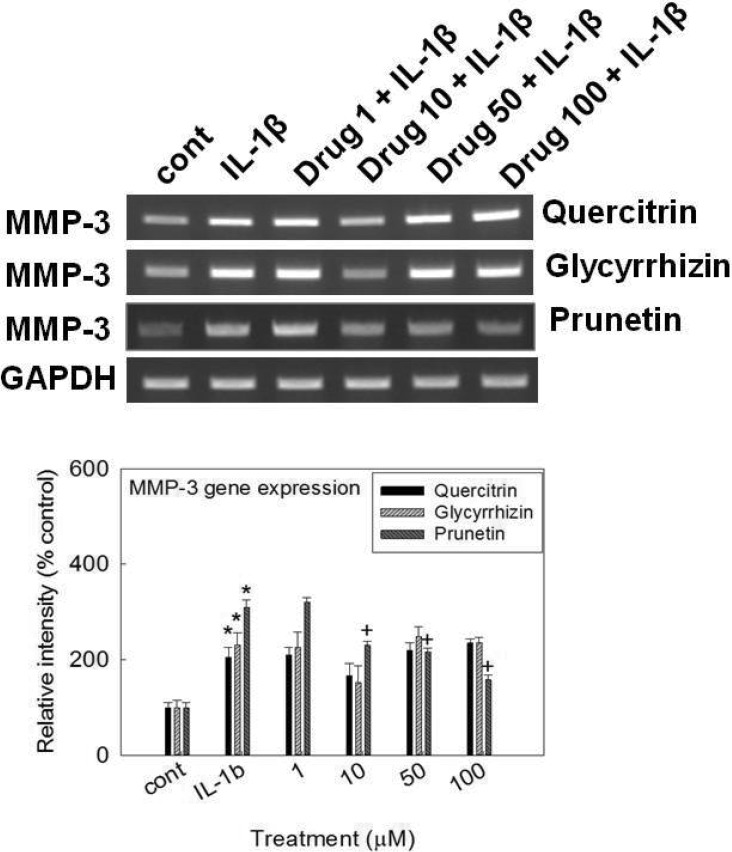
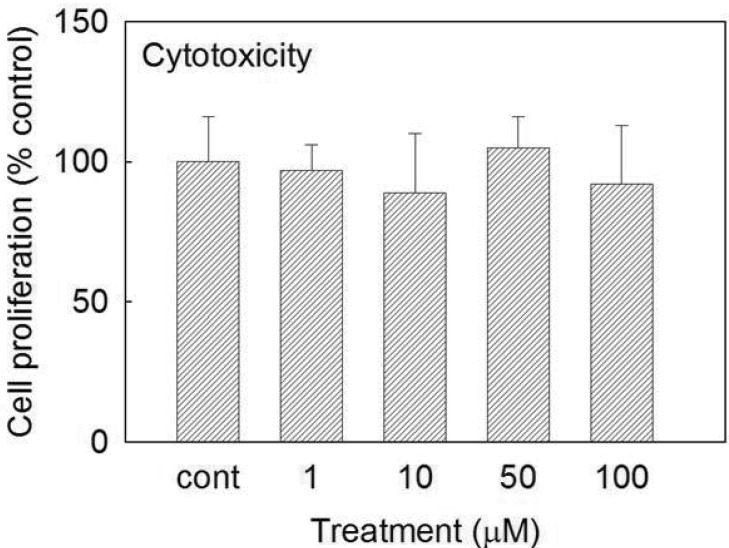
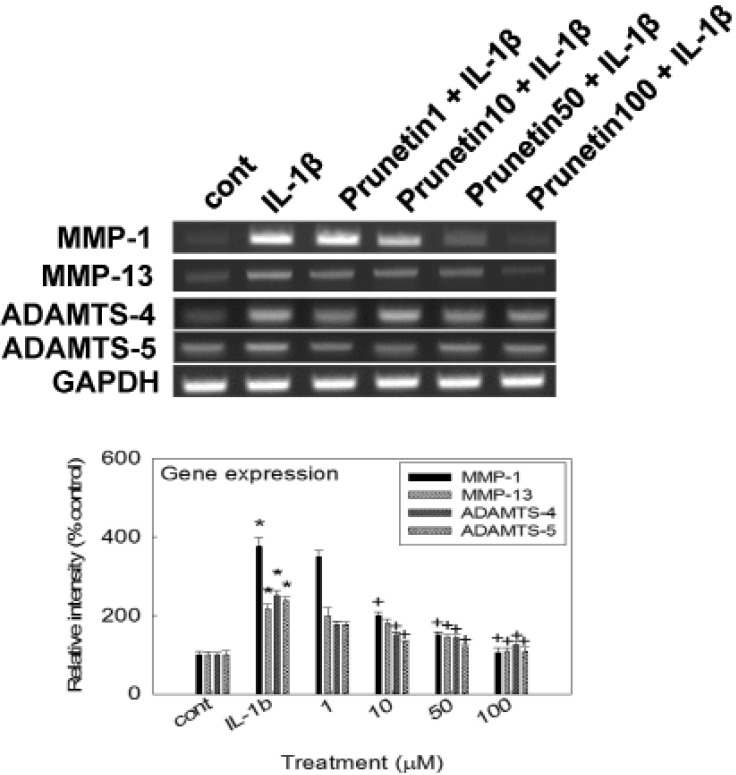
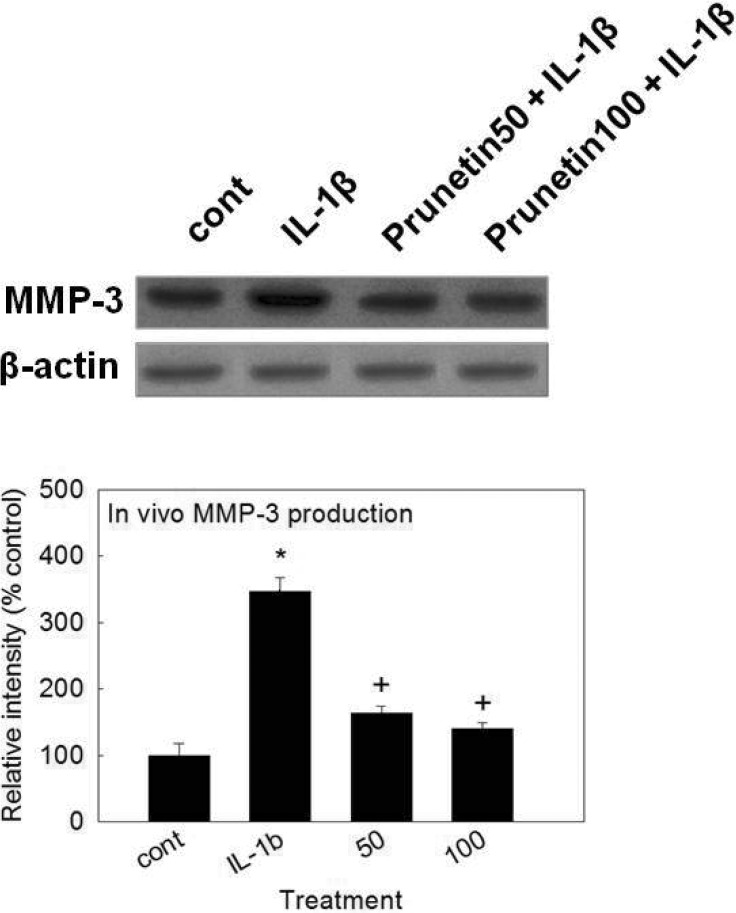
- We investigated whether prunetin affects the proteolytic activity, secretion, and gene expression of matrix metalloproteinase-3 (MMP-3) in primary cultured rabbit articular chondrocytes, as well as in vivo production of MMP-3 in the rat knee joint to evaluate the potential chondroprotective eff ect of prunetin. Rabbit articular chondrocytes were cultured in a monolayer, and reverse transcription-polymerase chain reaction (RT-PCR) was used to measure interleukin-1beta (IL-1beta)-induced expression of MMP-3, MMP-1, MMP-13, a disintegrin and metalloproteinase with thrombospondin motifs-4 (ADAMTS-4), and ADAMTS-5. In rabbit articular chondrocytes, the effects of prunetin on IL-1beta-induced secretion and proteolytic activity of MMP-3 were investigated using western blot analysis and casein zymography, respectively. The eff ect of prunetin on MMP-3 protein production was also examined in vivo. The results were as follows: (1) prunetin inhibited the gene expression of MMP-3, MMP-1, MMP-13, ADAMTS-4, and ADAMTS-5; (2) prunetin inhibited the secretion and proteolytic activity of MMP-3; (3) prunetin suppressed the production of MMP-3 protein in vivo. These results suggest that prunetin can regulate the gene expression, secretion, and proteolytic activity of MMP-3, by directly acting on articular chondrocytes.
Keyword
MeSH Terms
Figure
Reference
-
1. Aigner T, Mckenna L. Molecular pathology and pathobiology of osteoarthritic cartilage. Cell Mol Life Sci. 2002; 59:5–18. PMID: 11846033.
Article2. Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982; 64:460–466. PMID: 6174527.
Article3. Dean D, Martel-pelletier J, Pelletier JP, Howell DS, Woessner JFJ. Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest. 1989; 84:678–685. PMID: 2760206.
Article4. Kullich W, Fagerer N, Schwann H. Effect of the NSAID nimesulide on the radical scavenger glutathione S-transferase in patients with osteoarthritis of the knee. Curr Med Res Opin. 2007; 23:1981–1986. PMID: 17631696.
Article5. Birkedal-hansen H, Moore WG, Bodden MK, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993; 4:197–250. PMID: 8435466.
Article6. Burrage P, Mix KS, Brinckerhoff CE. Matrix metal-loproteinases: role in arthritis. Front Biosci. 2006; 11:529–543. PMID: 16146751.
Article7. Garnero P, Rousseau JC, Delmas PD. Molecular basis and clinical use of biochemical markers of bone, cartilage, and synovium in joint diseases. Arthritis Rheum. 2000; 43:953–968. PMID: 10817547.
Article8. Lin P, Chen CT, Torzilli PA. Increased stromelysin-1 (MMP-3), proteoglycan degradation (3B3- and 7D4) and collagen damage in cyclically load-injured articular cartilage. Osteoarthritis Cartilage. 2004; 12:485–496. PMID: 15135145.
Article9. Yang G, Ham I, Choi HY. Anti-inflammatory effect of prunetin via the suppression of NF-κB pathway. Food Chem Toxicol. 2013; 58:124–132. PMID: 23597450.
Article10. Lee HJ, Lee SY, Lee MN, Kim JH, Chang GT, Seok JH, Lee CJ. Inhibition of secretion, production and gene expression of mucin from cultured airway epithelial cells by prunetin. Phytother Res. 2011; 25:1196–1200. PMID: 21305630.
Article11. Ryu J, Lee HJ, Park SH, Sikder MA, Kim JO, Hong JH, Seok JH, Lee CJ. Effect of prunetin on TNF-α-induced MUC5AC mucin gene expression, production, degradation of IκB and translocation of NF-κB p65 in human airway epithelial cells. Tuberc Respir Dis. 2013; 75:205–209.
Article12. Ahn TG, Yang G, Lee HM, Kim MD, Choi HY, Park KS, Lee SD, Kook YB, An HJ. Molecular mechanisms underlying the anti-obesity potential of prunetin, an O-methylated isoflavone. Biochem Pharmacol. 2013; 85:1525–1533. PMID: 23438470.
Article13. Sheikh S, Weiner H. Allosteric inhibition of human liver aldehyde dehydrogenase by the isoflavone prunetin. Biochem Pharmacol. 1997; 53:471–478. PMID: 9105397.
Article14. Moon PD, Jeong HS, Chun CS, Kim HM. Baekjeolyusin-tang and its active component berberine block the release of collagen and proteoglycan from IL-1β-stimulated rabbit cartilage and down-regulate matrix metalloproteinases in rabbit chondrocytes. Phytother Res. 2011; 25:844–850. PMID: 21089182.
Article15. Skehan P1, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990; 82:1107–1112. PMID: 2359136.
Article16. Bonnet C, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford). 2005; 44:7–16. PMID: 15292527.
Article17. Goldring MB, Otero M, Tsuchimochi K, Ijiri K, Li Y. Defining the roles of inflammatory and anabolic cytokines in cartilage metabolism. Ann Rheum Dis. 2008; 67(Suppl 3):iii75–iii82. PMID: 19022820.
Article18. Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, Feige U, Poole AR. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005; 52:128–135. PMID: 15641080.19. Loeser RF. Molecular mechanisms of cartilage destruction: mechanics, inflammatory mediators, and aging collide. Arthritis Rheum. 2006; 54:1357–1360. PMID: 16645963.
Article20. Aida Y, Maeno M, Suzuki N, Shiratsuchi H, Motohashi M, Matsumura H. The effect of IL-1beta on the expression of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in human chondrocytes. Life Sci. 2005; 77:3210–3221. PMID: 15979654.21. Pantsulaia I, Kalichman L, Kobyliansky E. Association between radiographic hand osteoarthritis and RANKL, OPG and inflammatory markers. Osteoarthritis Cartilage. 2010; 18:1448–1453. PMID: 20633673.
Article22. Lijnen HR. Matrix metalloproteinases and cellular fibrinolytic activity. Biochemistry Mosc. 2002; 67:92–98. PMID: 11841344.23. Freemont AJ, Hampson V, Tilman R, Goupille P, Taiwo Y, Hoyland JA. Gene expression of matrix metalloproteinases 1, 3, and 9 by chondrocytes in osteoarthritic human knee articular cartilage is zone and grade specific. Ann Rheum Dis. 1997; 56:542–549. PMID: 9370879.
Article24. Goupille P, Jayson MI, Valat JP, Freemont AJ. Matrix metalloproteinases: the clue to intervertebral disc degeneration? Spine (Phila Pa 1976). 1998; 23:1612–1626. PMID: 9682320.
Article25. Kanyama M, Kuboki T, Kojima S, Fujisawa T, Hattori T, Takigawa M, Yamashita A. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids of patients with temporomandibular joint osteoarthritis. J Orofac Pain. 2000; 14:20–30. PMID: 11203734.26. Jo H, Park JS, Kim EM, Jung MY, Lee SH, Seong SC, Park SC, Kim HJ, Lee MC. The in vitro effects of dehydroepiandrosterone on human osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2003; 11:585–594. PMID: 12880581.
Article27. Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, Shah M, Thompson EW, Little C, Barai A, Burkhardt D, et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009; 60:3723–3733. PMID: 19950295.
Article28. Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, Turner J, Wu W, Billinghurst C, Meijers T, Poole AR, Babij P, DeGennaro LJ. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001; 107:35–44. PMID: 11134178.
Article29. Yoshihara Y, Nakamura H, Obata K, Yamada H, Hayakawa T, Fujikawa K, Okada Y. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis. 2000; 59:455–461. PMID: 10834863.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Apigenin Regulates Interleukin-1β-Induced Production of Matrix Metalloproteinase Both in the Knee Joint of Rat and in Primary Cultured Articular Chondrocytes
- Effect of oleanolic acid on the activity, secretion and gene expression of matrix metalloproteinase-3 in articular chondrocytes in vitro and the production of matrix metalloproteinase-3 in vivo
- Chondroprotective Effects of Wogonin in Experimental Models of Osteoarthritis in vitro and in vivo
- Betulin suppressed interleukin-1β-induced gene expression, secretion and proteolytic activity of matrix metalloproteinase in cultured articular chondrocytes and production of matrix metalloproteinase in the knee joint of rat
- Luteolin Inhibits the Activity, Secretion and Gene Expression of MMP-3 in Cultured Articular Chondrocytes and Production of MMP-3 in the Rat Knee