Korean J Physiol Pharmacol.
2016 Mar;20(2):161-168. 10.4196/kjpp.2016.20.2.161.
CRM1 inhibitor S109 suppresses cell proliferation and induces cell cycle arrest in renal cancer cells
- Affiliations
-
- 1Insititute of Nervous System Diseases, Xuzhou Medical College, Xuzhou 221002, Jiangsu, China.
- 2Blood Disease Institute, Xuzhou Medical College, Xuzhou 221002, Jiangsu, China. msniu24@hotmail.com
- 3Department of Hematology, the Affiliated Hospital of Xuzhou Medical College, Xuzhou 221002, Jiangsu, China.
- 4Brain Hospital, the Affiliated Hospital of Xuzhou Medical College, Xuzhou 221002, Jiangsu, China.
- 5Dalian Center for Disease Control and Prevention, Dalian 116002, Liaoning, China.
- KMID: 2388672
- DOI: http://doi.org/10.4196/kjpp.2016.20.2.161
Abstract
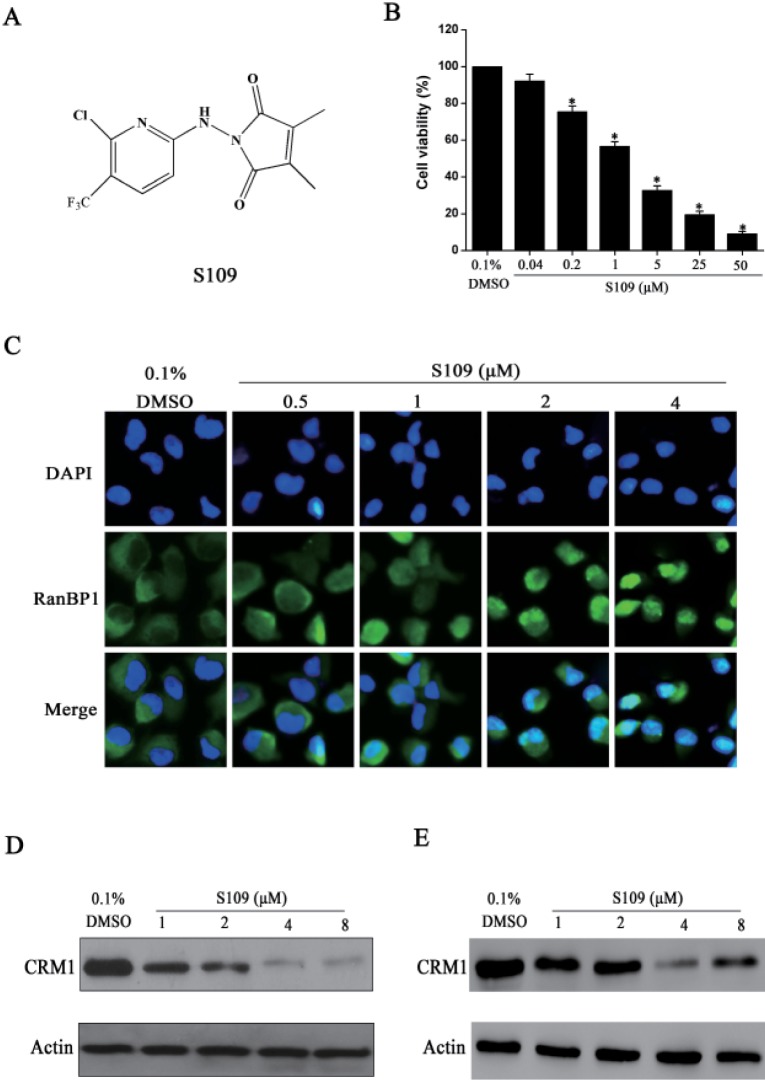
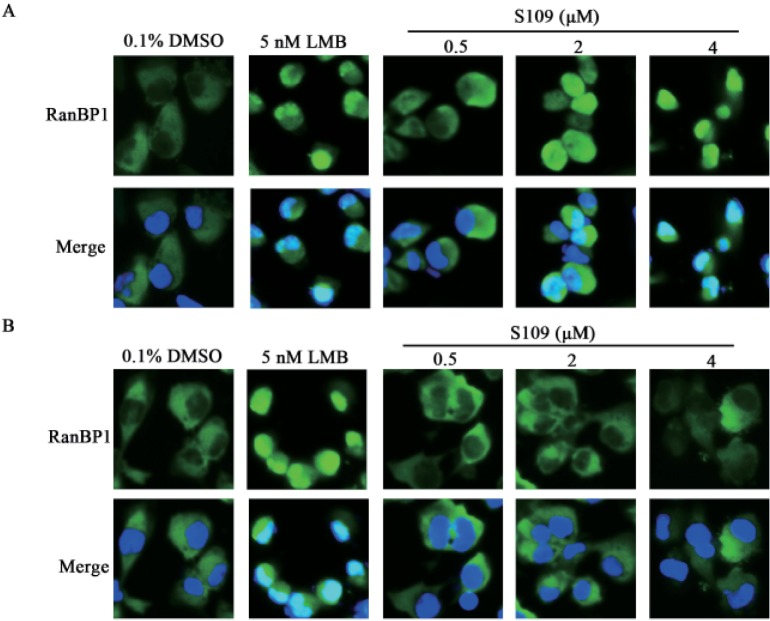
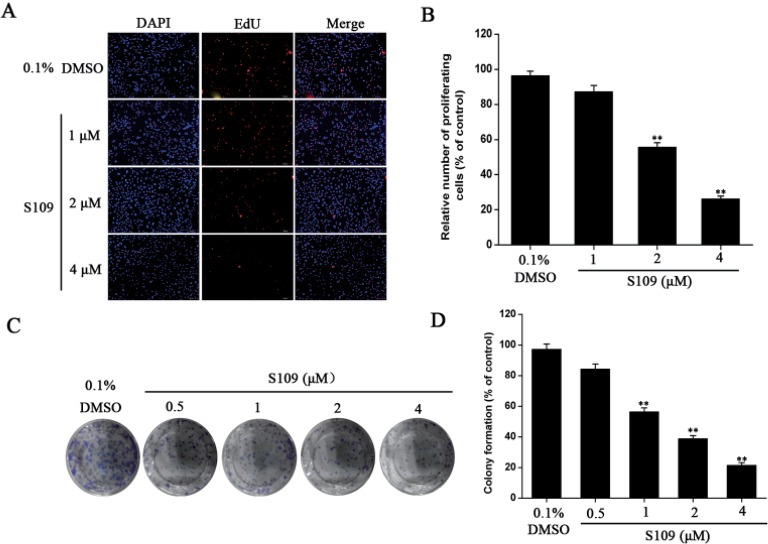
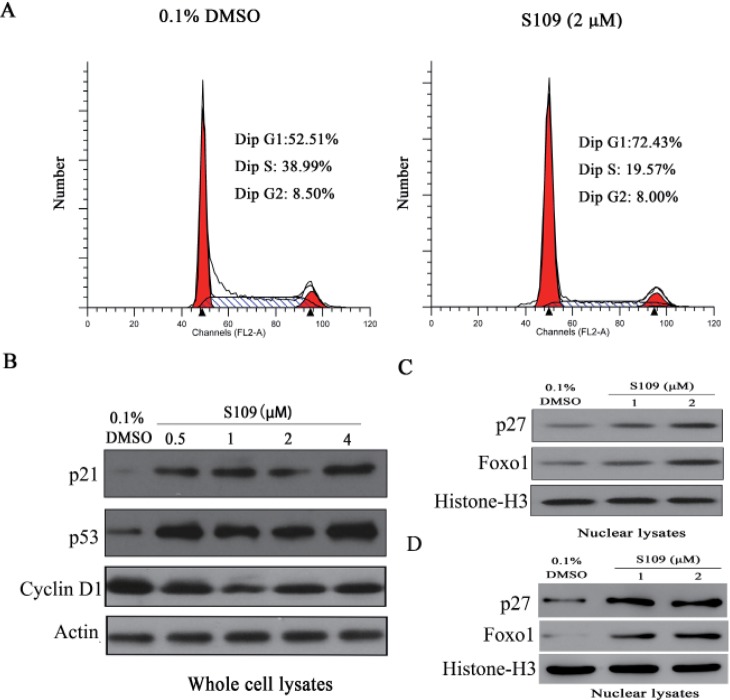
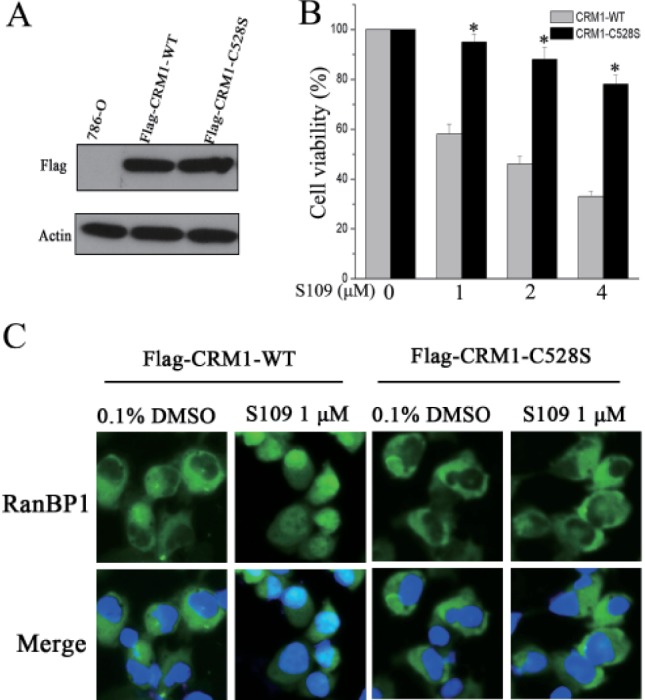
- Abnormal localization of tumor suppressor proteins is a common feature of renal cancer. Nuclear export of these tumor suppressor proteins is mediated by chromosome region maintenance-1 (CRM1). Here, we investigated the antitumor eff ects of a novel reversible inhibitor of CRM1 on renal cancer cells. We found that S109 inhibits the CRM1-mediated nuclear export of RanBP1 and reduces protein levels of CRM1. Furthermore, the inhibitory eff ect of S109 on CRM1 is reversible. Our data demonstrated that S109 signifi cantly inhibits proliferation and colony formation of renal cancer cells. Cell cycle assay showed that S109 induced G1-phase arrest, followed by the reduction of Cyclin D1 and increased expression of p53 and p21. We also found that S109 induces nuclear accumulation of tumor suppressor proteins, Foxo1 and p27. Most importantly, mutation of CRM1 at Cys528 position abolished the eff ects of S109. Taken together, our results indicate that CRM1 is a therapeutic target in renal cancer and the novel reversible CRM1 inhibitor S109 can act as a promising candidate for renal cancer therapy.
Keyword
MeSH Terms
Figure
Reference
-
1. Novara G, Ficarra V, Antonelli A, Artibani W, Bertini R, Carini M, Cosciani Cunico S, Imbimbo C, Longo N, Martignoni G, Martorana G, Minervini A, Mirone V, Montorsi F, Schiavina R, Simeone C, Serni S, Simonato A, Siracusano S, Volpe A, Carmignani G. SATURN Project-LUNA Foundation. Validation of the 2009 TNM version in a large multi-institutional cohort of patients treated for renal cell carcinoma: are further improvements needed? Eur Urol. 2010; 58:588–595. PMID: 20674150.
Article2. Hadoux J, Vignot S, De La Motte Rouge T. Renal cell carcinoma: focus on safety and efficacy of temsirolimus. Clin Med Insights Oncol. 2010; 4:143–154. PMID: 21234295.
Article3. Cho IC, Chung J. Current status of targeted therapy for advanced renal cell carcinoma. Korean J Urol. 2012; 53:217–228. PMID: 22536463.
Article4. Zhan YH, Liu J, Qu XJ, Hou KZ, Wang KF, Liu YP, Wu B. β-Elemene induces apoptosis in human renal-cell carcinoma 786-0 cells through inhibition of MAPK/ERK and PI3K/Akt/ mTOR signalling pathways. Asian Pac J Cancer Prev. 2012; 13:2739–2744. PMID: 22938451.5. Edeline J, Vigneau C, Patard JJ, Rioux-Leclercq N. Signalling pathways in renal-cell carcinoma: from the molecular biology to the future therapy. Bull Cancer. 2010; 97:5–15.6. Inoue H, Hwang SH, Wecksler AT, Hammock BD, Weiss RH. Sorafenib attenuates p21 in kidney cancer cells and augments cell death in combination with DNA-damaging chemotherapy. Cancer Biol Ther. 2011; 12:827–836. PMID: 21878748.
Article7. Voss MH, Molina AM, Motzer RJ. mTOR inhibitors in advanced renal cell carcinoma. Hematol Oncol Clin North Am. 2011; 25:835–852. PMID: 21763970.8. Su D, Stamatakis L, Singer EA, Srinivasan R. Renal cell carcinoma: molecular biology and targeted therapy. Curr Opin Oncol. 2014; 26:321–327. PMID: 24675233.9. Daelemans D, Costes SV, Lockett S, Pavlakis GN. Kinetic and molecular analysis of nuclear export factor CRM1 association with its cargo in vivo. Mol Cell Biol. 2005; 25:728–739. PMID: 15632073.
Article10. Turner JG, Sullivan DM. CRM1-mediated nuclear export of proteins and drug resistance in cancer. Curr Med Chem. 2008; 15:2648–2655. PMID: 18991627.
Article11. Xu D, Farmer A, Chook YM. Recognition of nuclear targeting signals by Karyopherin-βproteins. Curr Opin Struct Biol. 2010; 20:782–790. PMID: 20951026.12. Kanai M, Hanashiro K, Kim SH, Hanai S, Boulares AH, Miwa M, Fukasawa K. Inhibition of Crm1-p53 interaction and nuclear export of p53 by poly(ADP-ribosyl)ation. Nat Cell Biol. 2007; 9:1175–1183. PMID: 17891139.
Article13. Connor MK, Kotchetkov R, Cariou S, Resch A, Lupetti R, Beniston RG, Melchior F, Hengst L, Slingerland JM. CRM1/Ran-mediated nuclear export of p27(Kip1) involves a nuclear export signal and links p27 export and proteolysis. Mol Biol Cell. 2003; 14:201–213. PMID: 12529437.14. Wang W, Budhu A, Forgues M, Wang XW. Temporal and spatial control of nucleophosmin by the Ran-Crm1 complex in centrosome duplication. Nat Cell Biol. 2005; 7:823–830. PMID: 16041368.
Article15. Inoue H, Kauffman M, Shacham S, Landesman Y, Yang J, Evans CP, Weiss RH. CRM1 blockade by selective inhibitors of nuclear export attenuates kidney cancer growth. J Urol. 2013; 189:2317–2326. PMID: 23079374.
Article16. Payton S. Kidney cancer: CRM1-a novel drug target for renal cell carcinoma? Nat Rev Urol. 2012; 9:672.17. Yao Y, Dong Y, Lin F, Zhao H, Shen Z, Chen P, Sun YJ, Tang LN, Zheng SE. The expression of CRM1 is associated with prognosis in human osteosarcoma. Oncol Rep. 2009; 21:229–235. PMID: 19082467.
Article18. Huang WY, Yue L, Qiu WS, Wang LW, Zhou XH, Sun YJ. Prognostic value of CRM1 in pancreas cancer. Clin Invest Med. 2009; 32:E315–E321. PMID: 20003838.19. Tai YT, Landesman Y, Acharya C, Calle Y, Zhong MY, Cea M, Tannenbaum D, Cagnetta A, Reagan M, Munshi AA, Senapedis W, Saint-Martin JR, Kashyap T, Shacham S, Kauffman M, Gu Y, Wu L, Ghobrial I, Zhan F, Kung AL, Schey SA, Richardson P, Munshi NC, Anderson KC. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia. 2014; 28:155–165. PMID: 23588715.
Article20. Shen A, Wang Y, Zhao Y, Zou L, Sun L, Cheng C. Expression of CRM1 in human gliomas and its significance in p27 expression and clinical prognosis. Neurosurgery. 2009; 65:153–159. PMID: 19574837.
Article21. Kudo N, Matsumori N, Taoka H, Fujiwara D, Schreiner EP, Wolff B, Yoshida M, Horinouchi S. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc Natl Acad Sci U S A. 1999; 96:9112–9117. PMID: 10430904.
Article22. Gerecitano J. SINE (selective inhibitor of nuclear export)--translational science in a new class of anti-cancer agents. J Hematol Oncol. 2014; 7:67. PMID: 25281264.
Article23. Sakakibara K, Saito N, Sato T, Suzuki A, Hasegawa Y, Friedman JM, Kufe DW, Vonhoff DD, Iwami T, Kawabe T. CBS9106 is a novel reversible oral CRM1 inhibitor with CRM1 degrading activity. Blood. 2011; 118:3922–3931. PMID: 21841164.
Article24. Liu X, Cai W, Niu M, Chong Y, Liu H, Hu W, Wang D, Gao S, Shi Q, Hu J, Zhou X, Yu R. Plumbagin induces growth inhibition of human glioma cells by downregulating the expression and activity of FOXM1. J Neurooncol. 2015; 121:469–477. PMID: 25528634.
Article25. Cui Y, Park JY, Wu J, Lee JH, Yang YS, Kang MS, Jung SC, Park JM, Yoo ES, Kim SH, Ahn Jo S, Suk K, Eun SY. Dieckol attenuates microglia-mediated neuronal cell death via erk, akt and nadph oxidase-mediated pathways. Korean J Physiol Pharmacol. 2015; 19:219–228. PMID: 25954126.
Article26. Kang YH, Jin JS, Son SM. Long term effect of high glucose and phosphate levels on the opg/rank/rankl/trail system in the progression of vascular calcification in rat aortic smooth muscle cells. Korean J Physiol Pharmacol. 2015; 19:111–118. PMID: 25729272.
Article27. Hilliard M, Frohnert C, Spillner C, Marcone S, Nath A, Lampe T, Fitzgerald DJ, Kehlenbach RH. The anti-inf lammatory prostaglandin 15-deoxy-delta(12,14)-PGJ2 inhibits CRM1-dependent nuclear protein export. J Biol Chem. 2010; 285:22202–22210. PMID: 20457605.28. Liu XJ, Niu MS, Xu XY, Cai W, Zeng LY, Zhou XP, Yu RT, Xu KL. CRM1 is a direct cellular target of the natural anti- cancer agent plumbagin. J Pharmacol Sci. 2014; 124:486–493. PMID: 24739265.29. Weiss RH, Lin PY. Kidney cancer: identification of novel targets for therapy. Kidney Int. 2006; 69:224–232. PMID: 16408110.
Article30. Gravina GL, Senapedis W, McCauley D, Baloglu E, Shacham S, Festuccia C. Nucleo-cytoplasmic transport as a therapeutic target of cancer. J Hematol Oncol. 2015; 7:85.
Article31. Mendonca J, Sharma A, Kim HS, Hammers H, Meeker A, De Marzo A, Carducci M, Kauffman M, Shacham S, Kachhap S. Selective inhibitors of nuclear export (SINE) as novel therapeutics for prostate cancer. Oncotarget. 2014; 5:6102–6112. PMID: 25026284.
Article32. Nguyen KT, Holloway MP, Altura RA. The CRM1 nuclear export protein in normal development and disease. Int J Biochem Mol Biol. 2012; 3:137–151. PMID: 22773955.33. Burris HA. Overcoming acquired resistance to anticancer therapy: focus on the PI3K/AKT/mTOR pathway. Cancer Chemoth Pharm. 2013; 71:829–842.
Article34. Lin F, Zhang PL, Yang XJ, Prichard JW, Lun M, Brown RE. Morphoproteomic and molecular concomitants of an overexpressed and activated mTOR pathway in renal cell carcinomas. Ann Clin Lab Sci. 2006; 36:283–293. PMID: 16951269.35. Bilbao PS, Boland R. Extracellular ATP regulates FoxO family of transcription factors and cell cycle progression through PI3K/Akt in MCF-7 cells. Biochim Biophys Acta. 2013; 1830:4456–4469. PMID: 23742826.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of chromosomal region maintenance 1 suppresses the migration and invasion of glioma cells via inactivation of the STAT3/MMP2 signaling pathway
- Selective B-RAF V600E Inhibitor PLX4032 Inhibits the Growth of Breast Cancer Cell Lines through Cell Cycle Arrest
- The Nedd8-activating enzyme inhibitor MLN4924 suppresses colon cancer cell growth via triggering autophagy
- Effects of p27 Overexpression on Head and Neck Squamous Cell Carcinoma Cell Lines
- Role of p21CIP1 as a determinant of SC-560 response in human HCT116 colon carcinoma cells