Transl Clin Pharmacol.
2017 Jun;25(2):63-66. 10.12793/tcp.2017.25.2.63.
Allopurinol-induced severe cutaneous adverse reactions: A report of three cases with the HLA-B*58:01 allele who underwent lymphocyte activation test
- Affiliations
-
- 1Department of Clinical Pharmacology, Inje University College of Medicine, Busan Paik Hospital, Busan 47392, Republic of Korea. phshinjg@gmail.com
- 2Department of Dermatology, Inje University College of Medicine, Busan Paik Hospital, Busan 47392, Republic of Korea.
- 3Department of Microbiology and Immunology, Inje University College of Medicine, Busan 47392, Republic of Korea.
- 4Department of Pharmacology and PharmacoGenomics Research Center, Inje University College of Medicine, Busan 47392, Republic of Korea.
- KMID: 2386762
- DOI: http://doi.org/10.12793/tcp.2017.25.2.63
Abstract
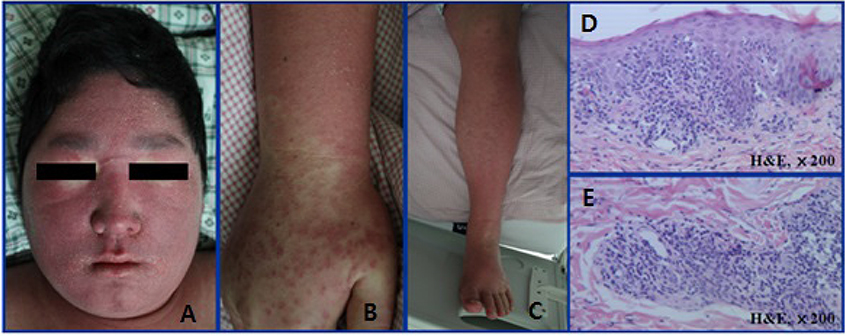
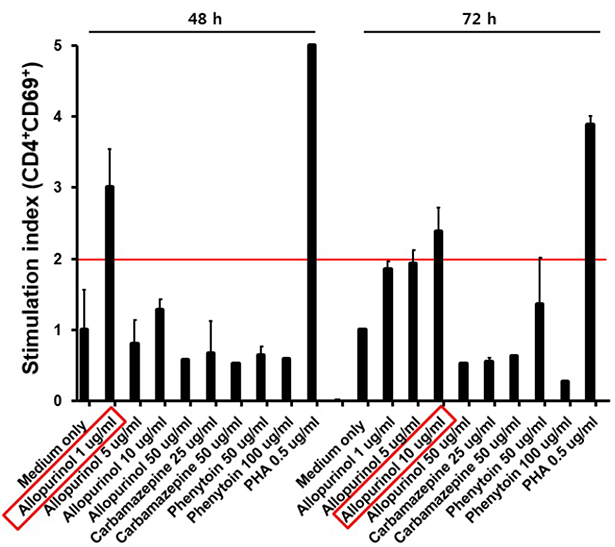
- Allopurinol-induced severe cutaneous adverse reactions (SCARs) such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome are reportedly associated with the HLA-B*58:01 genotype. Three patients who developed SCARs after allopurinol administration were subjected to HLA-B genotyping and lymphocyte activation test (LAT) to evaluate genetic risk and to detect the causative agent, respectively. All three patients given allopurinol to treat gout were diagnosed with DRESS syndrome. Symptom onset commenced 7-24 days after drug exposure; the patients took allopurinol (100-200 mg/d) for 2-30 days. HLA-B genotyping was performed using a polymerase chain reaction (PCR)-sequence-based typing (SBT) method. All patients had a single HLA-B*58:01 allele: HLA-B*13:02/*58:01 (a 63-year-old male), HLA-B*48:01/*58:01 (a 71-year-old female), and HLA-B*44:03/*58:01 (a 22-year-old male). Only the last patient yielded a positive LAT result, confirming that allopurinol was the causative agent. These findings suggest that patients with HLA-B*58:01 may develop SCARs upon allopurinol administration. Therefore, HLA-B genotyping could be helpful in preventing serious problems attributable to allopurinol treatment, although PCR-SBT HLA-B genotyping is time consuming. A simple genotyping test is required in practice. LAT may help to identify a causative agent.
Keyword
MeSH Terms
Figure
Reference
-
1. Pirmohamed M, Friedmann PS, Molokhia M, Loke YK, Smith C, Phillips E, et al. Phenotype standardization for immune-mediated drug-induced skin injury. Clin Pharmacol Ther. 2011; 89:896–901. DOI: 10.1038/clpt.2011.79.
Article2. Mallal S, Nolan D, Witt C, Masel G, Martin AM, Moore C, et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. 2002; 359:727–732.
Article3. Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, et al. Medical genetics: A marker for Stevens-Johnson syndrome. Nature. 2004; 428:486.4. Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci USA. 2005; 102:4134–4139.
Article5. Kaniwa N, Saito Y, Aihara M, Matsunaga K, Tohkin M, Kurose K, et al. HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics. 2008; 9:1617–1622. DOI: 10.2217/14622416.9.11.1617.
Article6. Kang HR, Jee YK, Kim YS, Lee CH, Jung JW, Kim SH, et al. Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharmacogenet Genomics. 2011; 21:303–307. DOI: 10.1097/FPC.0b013e32834282b8.
Article7. Romano A, Torres MJ, Castells M, Sanz ML, Blanca M. Diagnosis and management of drug hypersensitivity reactions. J Allergy Clin Immunol. 2011; 127:S67–S73. DOI: 10.1016/j.jaci.2010.11.047.
Article8. Kim MH, Shim EJ, Jung JW, Sohn SW, Kang HR. A case of allopurinol-induced fixed drug eruption confirmed with a lymphocyte transformation test. Allergy Asthma Immunol Res. 2012; 4:309–310. DOI: 10.4168/aair.2012.4.5.309.
Article9. Beeler A, Zaccaria L, Kawabata T, Gerber BO, Pichler WJ. CD69 upregulation on T cells as an in vitro marker for delayed-type drug hypersensitivity. Allergy. 2008; 63:181–188.
Article10. Ng CY, Yeh YT, Wang CW, Hung SI, Yang CH, Chang YC, et al. Impact of the HLA-B(*)58:01 allele and renal impairment on allopurinol-induced cutaneous adverse reactions. J Invest Dermatol. 2016; 136:1373–1381. DOI: 10.1016/j.jid.2016.02.808.
Article11. Jutkowitz E, Dubreuil M, Lu N, Kuntz KM, Choi HK. The cost-effectiveness of HLA-B*5801 screening to guide initial urate-lowering therapy for gout in the United States. Semin Arthritis Rheum. 2017; 46:594–600. DOI: 10.1016/j.semarthrit.2016.10.009.
Article12. In JW, Roh EY, Oh S, Shin S, Park KU, Song EY. Allele and haplotype frequencies of human leukocyte antigen-A, -B, -C, -DRB1, and -DQB1 from sequence-based DNA typing data in Koreans. Ann Lab Med. 2015; 35:429–435. DOI: 10.3343/alm.2015.35.4.429.
Article13. Lochmatter P, Beeler A, Kawabata TT, Gerber BO, Pichler WJ. Drug-specific in vitro release of IL-2, IL-5, IL-13, and IFN-gamma in patients with delayed-type drug hypersensitivity. Allergy. 2009; 64:1269–1278. DOI: 10.1111/j.1398-9995.2009.01985.x.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Validation of a Rapid, Robust, Inexpensive Screening Method for Detecting the HLA-B*58:01 Allele in the Prevention of Allopurinol-Induced Severe Cutaneous Adverse Reactions
- HLA Allele Frequencies in 5802 Koreans: Varied Allele Types Associated with SJS/TEN According to Culprit Drugs
- Antiepileptic drug-induced severe cutaneous adverse reactions and HLA alleles: A report of five cases with lymphocyte activation test
- Genetic markers of severe cutaneous adverse reactions
- Hypersensitivity to Anticonvulsants