Allergy Asthma Immunol Res.
2017 Jan;9(1):79-84. 10.4168/aair.2017.9.1.79.
Validation of a Rapid, Robust, Inexpensive Screening Method for Detecting the HLA-B*58:01 Allele in the Prevention of Allopurinol-Induced Severe Cutaneous Adverse Reactions
- Affiliations
-
- 1Sydney Medical School - Northern, University of Sydney, Sydney, Australia. vngu3162@uni.sydney.edu.au
- 2ImmunoRheumatology Laboratory, Pathology North-Northern Sydney, St Leonards, Australia. christophervid@gmail.com
- 3Department of Allergy and Clinical Immunology, Hanoi Medical University, Hanoi, Vietnam.
- 4Center of Allergology and Clinical Immunology, Bach Mai Hospital, Hanoi, Vietnam.
- 5Department of Clinical Immunology and Allergy, Royal North Shore Hospital, Sydney, Australia.
- KMID: 2355897
- DOI: http://doi.org/10.4168/aair.2017.9.1.79
Abstract
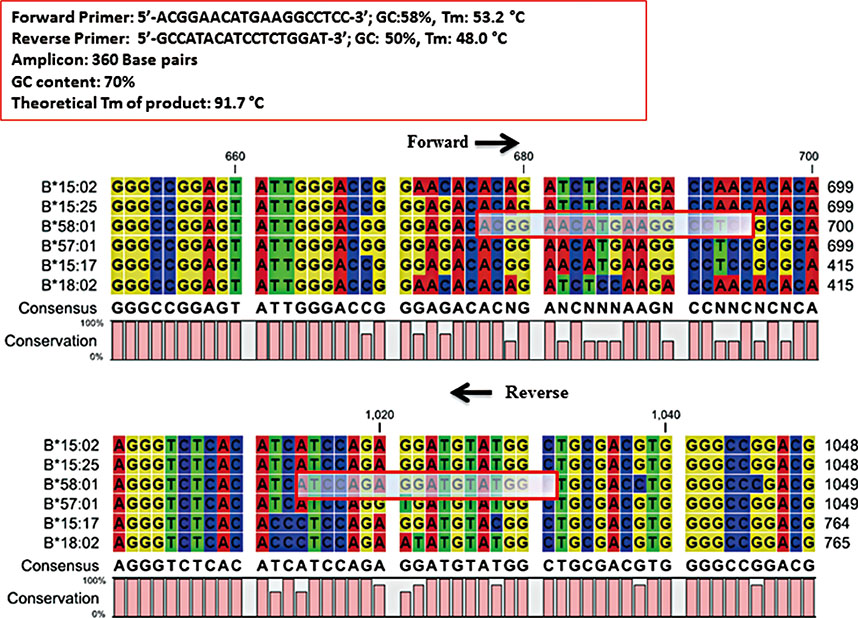
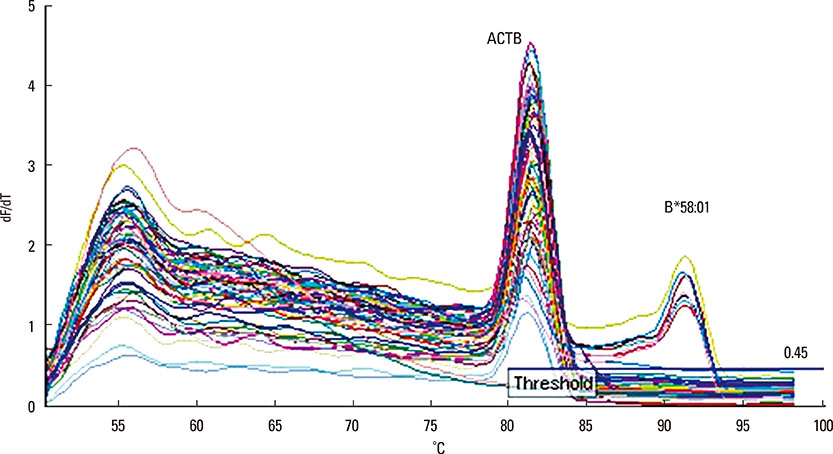
- The HLA B*58:01 allele has been worldwide reported as a pharmacogenetic susceptibility to allopurinol-induced severe cutaneous adverse reactions (SCARs). To prevent these life-threatening conditions, the American College of Rheumatology hingly recommended that the HLA-B*58:01 be screened prior to the initiation of allopurinol therapy. Therefore, we developed a rapid, robust, inexpensive screening method using SYBR® Green real time PCR to detect the HLA-B*58:01 allele. A total of 119 samples were tested. The assay has a sensitivity of 100% (95% CI: 69.15%-100%), a specificity of 100% (95% CI: 96.67%-100%), a positive predictive value of 100% (95% CI: 69.15%-100%) and a negative predictive value of 100% (95% CI: 96.67%-100%). HLA-B*58:01 genotyping results showed 100% agreement with those obtained from Luminex SSO/SBT/SSP. The lowest limit of detection of this method is 0.8 ng/µL of DNA. The unit cost of the test is only $3.8 USD. This novel screening test using SYBR® real time PCR would be appropriate to identify individuals with the HLA-B*58:01 allele for the prevention of allopurinol-induced SCARs.
Keyword
MeSH Terms
Figure
Reference
-
1. Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012; 64:1431–1446.2. Ramasamy SN, Korb-Wells CS, Kannangara DR, Smith MW, Wang N, Roberts DM, et al. Allopurinol hypersensitivity: a systematic review of all published cases, 1950-2012. Drug Saf. 2013; 36:953–980.3. Zhang X, Ma H, Hu C, Yu B, Ma W, Wu Z, et al. Detection of HLA-B*58:01 with TaqMan assay and its association with allopurinol-induced sCADR. Clin Chem Lab Med. 2015; 53:383–390.4. Halevy S, Ghislain PD, Mockenhaupt M, Fagot JP, Bouwes Bavinck JN, Sidoroff A, et al. Allopurinol is the most common cause of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe and Israel. J Am Acad Dermatol. 2008; 58:25–32.5. Hung SI, Chung WH, Chen YT. HLA-B genotyping to detect carbamazepine-induced Stevens-Johnson syndrome: implications for personalizing medicine. Per Med. 2005; 2:225–237.6. Somkrua R, Eickman EE, Saokaew S, Lohitnavy M, Chaiyakunapruk N. Association of HLA-B*5801 allele and allopurinol-induced Stevens Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. BMC Med Genet. 2011; 12:118.7. Tassaneeyakul W, Jantararoungtong T, Chen P, Lin PY, Tiamkao S, Khunarkornsiri U, et al. Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet Genomics. 2009; 19:704–709.8. Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005; 102:4134–4139.9. Saokaew S, Tassaneeyakul W, Maenthaisong R, Chaiyakunapruk N. Cost-effectiveness analysis of HLA-B*5801 testing in preventing allopurinol-induced SJS/TEN in Thai population. PLoS One. 2014; 9:e94294.10. Ko TM, Tsai CY, Chen SY, Chen KS, Yu KH, Chu CS, et al. Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study. BMJ. 2015; 351:h4848.11. Park DJ, Kang JH, Lee JW, Lee KE, Wen L, Kim TJ, et al. Cost-effectiveness analysis of HLA-B5801 genotyping in the treatment of gout patients with chronic renal insufficiency in Korea. Arthritis Care Res (Hoboken). 2015; 67:280–287.12. Pavlos R, Mallal S, Phillips E. HLA and pharmacogenetics of drug hypersensitivity. Pharmacogenomics. 2012; 13:1285–1306.13. Kang X, Chen R, Han M, Liu Z, Liu J, Dai P, et al. Rapid and reliable genotyping of HLA-B*58:01 in four Chinese populations using a single-tube duplex real time PCR assay. Pharmacogenomics. 2016; 17:47–57.14. Yeo SI. HLA-B*5801: utility and cost-effectiveness in the Asia-Pacific Region. Int J Rheum Dis. 2013; 16:254–257.15. Nguyen DV, Vidal C, Li J, Fulton RB, Fernando SL. Validation of a rapid test for HLA-B*58:01/57:01 allele screening to detect individuals at risk for drug-induced hypersensitivity. Pharmacogenomics. 2016; 17:473–480.16. Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988; 16:1215.17. Virakul S, Nakkuntod J, Kupatawintu P, Kangwanshiratada O, Paiboonkasarp S, Hirankarn N. Detection of HLA-B*5801 by in-house PCR-SSP. In : The 11th Graduate Research Conference, Khonkaen University; Khon Kaen: Khonkaen University;2010. p. 960–966.18. Kwok J, Kwong KM. Detection of HLA-B*58:01, the susceptible allele for allopurinol-induced hypersensitivity, by loop-mediated isothermal amplification. Br J Dermatol. 2013; 168:526–532.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Allopurinol-induced severe cutaneous adverse reactions: A report of three cases with the HLA-B*58:01 allele who underwent lymphocyte activation test
- HLA Allele Frequencies in 5802 Koreans: Varied Allele Types Associated with SJS/TEN According to Culprit Drugs
- Genetic markers of severe cutaneous adverse reactions
- Antiepileptic drug-induced severe cutaneous adverse reactions and HLA alleles: A report of five cases with lymphocyte activation test
- Hypersensitivity to Anticonvulsants