Transl Clin Pharmacol.
2017 Jun;25(2):59-62. 10.12793/tcp.2017.25.2.59.
Pharmacokinetic variability due to environmental differences
- Affiliations
-
- 1Department of Pharmacology & Clinical Pharmacology, University of Auckland, Auckland, New Zealand. n.holford@auckland.ac.nz
- KMID: 2386761
- DOI: http://doi.org/10.12793/tcp.2017.25.2.59
Abstract
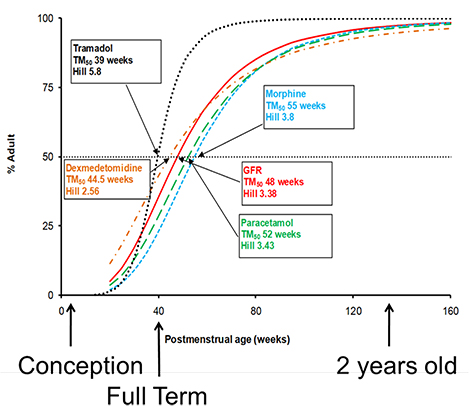
- This tutorial describes sources of pharmacokinetic variability that are not obviously linked to genetic differences. The sources of variability are therefore described as environmental. The major quantitative sources of environmental variability are body size (including body composition), maturation and organ function. Size should be considered in all patients. Maturation is mainly relevant to neonates and infants less than 2 years of age. Renal function is the most important predictable source of variability due to differences in organ function.
Keyword
Figure
Cited by 1 articles
-
Pharmacodynamic principles and target concentration intervention
Nick Holford
Transl Clin Pharmacol. 2018;26(4):150-154. doi: 10.12793/tcp.2018.26.4.150.
Reference
-
1. West GB, Brown JH, Enquist BJ. The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science. 1999; 284:1677–1679.
Article2. Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005; 44:1051–1065.
Article3. Xue L, Holford N, Ding XL, Shen ZY, Huang CR, Zhang H, et al. Theory-based pharmacokinetics and pharmacodynamics of S- and R-warfarin and effects on international normalized ratio: influence of body size, composition and genotype in cardiac surgery patients. Br J Clin Pharmacol. 2017; 83:823–835. DOI: 10.1111/bcp.13157.
Article4. McCune JS, Bemer MJ, Barrett JS, Scott Baker K, Gamis AS, Holford NH. Busulfan in Infant to Adult Hematopoietic Cell Transplant Recipients: A Population Pharmacokinetic Model for Initial and Bayesian Dose Personalization. Clin Cancer Res. 2014; 20:754–763. DOI: 10.1158/1078-0432.CCR-13-1960.
Article5. Cortínez LI, Anderson BJ, Penna A, Olivares L, Muñoz HR, Holford NH, et al. Influence of obesity on propofol pharmacokinetics: derivation of a pharmacokinetic model. Br J Anaesth. 2010; 105:448–456. DOI: 10.1093/bja/aeq195.
Article6. Holford NHG, Anderson BJ. Allometric size: The scientific theory and extension to normal fat mass. Eur J Pharm Sci. 2017; DOI: 10.1016/j.ejps.2017.05.056.
Article7. Schwartz GJ. Does kL/PCr estimate GFR, or does GFR determine k? Pediatr Nephrol. 1992; 6:512–515.
Article8. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999; 130:461–470.
Article9. Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009; 24:67–76. DOI: 10.1007/s00467-008-0997-5.
Article10. Anderson BJ, Holford NHG. Tips and traps analyzing pediatric PK data. Paediatr Anaesth. 2011; 21:222–237. DOI: 10.1111/j.1460-9592.2011.03536.x.
Article11. Potts L, Anderson BJ, Warman GR, Lerman J, Diaz SM, Vilo S. Dexmedetomidine pharmacokinetics in pediatric intensive care; a pooled analysis. Pediatric Anesthesia. 2009; 19:1119–1129. DOI: 10.1111/j.1460-9592.2009.03133.x.
Article12. Allegaert K, Holford N, Anderson BJ, Holford S, Stuber F, Rochette A, et al. Tramadol and O-desmethyl tramadol clearance maturation and disposition in humans: a pooled pharmacokinetic study. Clin Pharmacokinet. 2015; 54:167–178. DOI: 10.1007/s40262-014-0191-9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Contributing Factors on Pharmacokinetic Variability in Critically Ill Neonates
- Pharmacogenetics of anesthetics
- Development of Physiologically Based Pharmacokinetic Model for Several Volatile Organic Compounds
- PKconverter: R package to convert the pharmacokinetic parameters
- Tacrolimus versus Cyclosporine Immunosuppression in Pediatric Renal Transplantation: Pharmacokinetic Consideration