Yonsei Med J.
2014 Mar;55(2):304-309.
In-Vitro Stem Cell Derived Red Blood Cells for Transfusion: Are We There Yet?
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea. hyunok1019@yuhs.ac
Abstract
- To date, the use of red blood cells (RBCs) produced from stem cells in vitro has not proved practical for routine transfusion. However, the perpetual and widespread shortage of blood products, problems related to transfusion-transmitted infections, and new emerging pathogens elicit an increasing demand for artificial blood. Worldwide efforts to achieve the goal of RBC production through stem cell research have received vast attention; however, problems with large-scale production and cost effectiveness have yet to prove practical usefulness. Some progress has been made, though, as cord blood stem cells and embryonic stem cells have shown an ability to differentiate and proliferate, and induced pluripotent stem cells have been shown to be an unlimited source for RBC production. However, transfusion of stem cell-derived RBCs still presents a number of challenges to overcome. This paper will summarize an up to date account of research and advances in stem cell-derived RBCs, delineate our laboratory protocol in producing RBCs from cord blood, and introduce the technological developments and limitations to current RBC production practices.
MeSH Terms
Figure
Reference
-
1. Giangrande PLF. The history of blood transfusion. Br J Haematol. 2000; 110:758–767.
Article2. Yamamoto F, Marken J, Tsuji T, White T, Clausen H, Hakomori S. Cloning and characterization of DNA complementary to human UDP-GalNAc: Fuc alpha 1----2Gal alpha 1----3GalNAc transferase (histo-blood group A transferase) mRNA. J Biol Chem. 1990; 265:1146–1151.
Article3. Ali A, Auvinen MK, Rautonen J. The aging population poses a global challenge for blood services. Transfusion. 2010; 50:584–588.4. Seifried E, Klueter H, Weidmann C, Staudenmaier T, Schrezenmeier H, Henschler R, et al. How much blood is needed? Vox Sang. 2011; 100:10–21.
Article5. Buemi M, Lacquaniti A, Bolignano D, Cernaro V, Campo S, Grasso G, et al. Down with the erythropoietin. Long live the erythropoietin! Curr Drug Targets. 2009; 10:1028–1032.6. Neildez-Nguyen TM, Wajcman H, Marden MC, Bensidhoum M, Moncollin V, Giarratana MC, et al. Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat Biotechnol. 2002; 20:467–472.
Article7. Giarratana MC, Kobari L, Lapillonne H, Chalmers D, Kiger L, Cynober T, et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005; 23:69–74.
Article8. Miharada K, Hiroyama T, Sudo K, Nagasawa T, Nakamura Y. Efficient enucleation of erythroblasts differentiated in vitro from hematopoietic stem and progenitor cells. Nat Biotechnol. 2006; 24:1255–1256.
Article9. Baek EJ, Kim HS, Kim S, Jin H, Choi TY, Kim HO. In vitro clinical-grade generation of red blood cells from human umbilical cord blood CD34+ cells. Transfusion. 2008; 48:2235–2245.
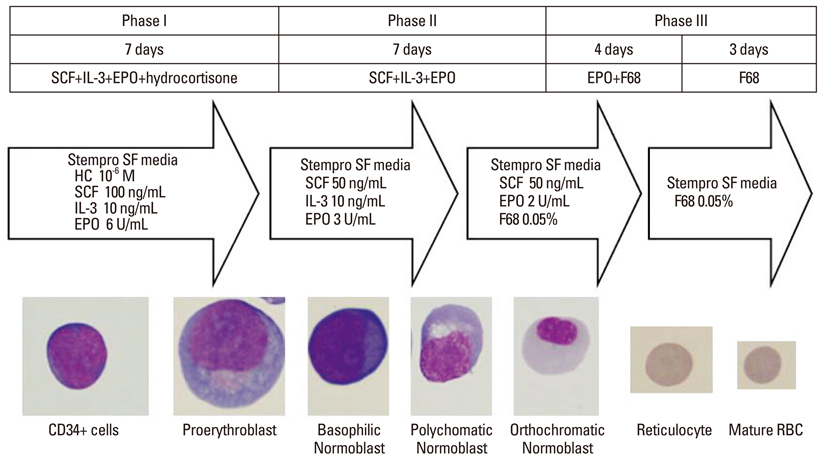
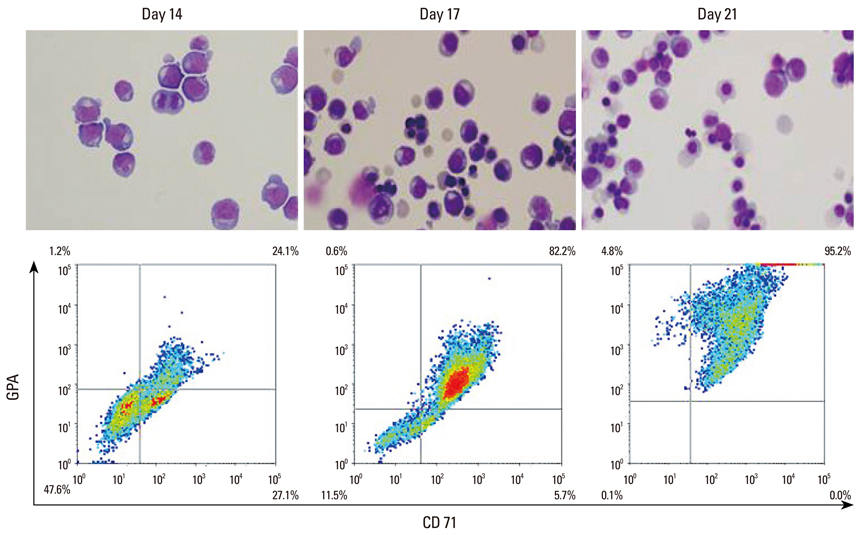
Article10. Baek EJ, Kim HS, Kim JH, Kim NJ, Kim HO. Stroma-free mass production of clinical-grade red blood cells (RBCs) by using poloxamer 188 as an RBC survival enhancer. Transfusion. 2009; 49:2285–2295.
Article11. Baek EJ, You J, Kim MS, Lee SY, Cho SJ, Kim E, et al. Enhanced production of red blood cells in suspension by electrostatic interactions with culture plates. Tissue Eng Part C Methods. 2010; 16:1325–1334.
Article12. Kim HO, Baek EJ. Red blood cell engineering in stroma and serum/plasma-free conditions and long term storage. Tissue Eng Part A. 2012; 18:117–126.
Article13. Koury MJ, Lichtman MA. Structure of the marrow and the hematopoietic microenvironment. In : Kaushansky K, Williams WJ, editors. Williams hematology. 8th ed. New York: McGraw-Hill Medical;2010. p. 41–73.14. Tsiftsoglou AS, Vizirianakis IS, Strouboulis J. Erythropoiesis: model systems, molecular regulators, and developmental programs. IUBMB Life. 2009; 61:800–830.
Article15. Boehm D, Murphy WG, Al-Rubeai M. The potential of human peripheral blood derived CD34+ cells for ex vivo red blood cell production. J Biotechnol. 2009; 144:127–134.
Article16. Giarratana MC, Rouard H, Dumont A, Kiger L, Safeukui I, Le Pennec PY, et al. Proof of principle for transfusion of in vitro-generated red blood cells. Blood. 2011; 118:5071–5079.
Article17. Douay L, Giarratana MC. Ex vivo generation of human red blood cells: a new advance in stem cell engineering. Methods Mol Biol. 2009; 482:127–140.
Article18. Mountford J, Olivier E, Turner M. Prospects for the manufacture of red cells for transfusion. Br J Haematol. 2010; 149:22–34.
Article19. Nakamura Y. In vitro production of transfusable red blood cells. Biotechnol Genet Eng Rev. 2008; 25:187–201.
Article20. Choi HS, Lee EM, Kim HO, Park MI, Baek EJ. Autonomous control of terminal erythropoiesis via physical interactions among erythroid cells. Stem Cell Res. 2013; 10:442–453.
Article21. Park KS, Ahn J, Kim JY, Park H, Kim HO, Lee SH. Poly-l-lysine increases the ex vivo expansion and erythroid differentiation of human hematopoietic stem cells, as well as erythroid enucleation efficacy. Tissue Eng Part A. 2014; 01. 24. [Epub ahead of print].22. Defense Advanced Research Projects Agency. Defense Sciences Office. Available at: http://www.darpa.mil/Our_Work/DSO/Programs/Blood_Pharming.aspx.23. DeWitt JC, Peden-Adams MM, Keller JM, Germolec DR. Immunotoxicity of perfluorinated compounds: recent developments. Toxicol Pathol. 2012; 40:300–311.
Article24. Timmins NE, Nielsen LK. Blood cell manufacture: current methods and future challenges. Trends Biotechnol. 2009; 27:415–422.
Article25. Keller G, Kennedy M, Papayannopoulou T, Wiles MV. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993; 13:473–486.
Article26. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998; 282:1145–1147.
Article27. Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2001; 98:10716–10721.
Article28. Ma F, Ebihara Y, Umeda K, Sakai H, Hanada S, Zhang H, et al. Generation of functional erythrocytes from human embryonic stem cell-derived definitive hematopoiesis. Proc Natl Acad Sci U S A. 2008; 105:13087–13092.
Article29. Mountford JC, Olivier E, Jordanides NE, de Sousa P, Turner ML. Red blood cells from pluripotent stem cells for use in transfusion. Regen Med. 2010; 5:411–423.
Article30. Lu SJ, Feng Q, Park JS, Vida L, Lee BS, Strausbauch M, et al. Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood. 2008; 112:4475–4484.
Article31. Lapillonne H, Kobari L, Mazurier C, Tropel P, Giarratana MC, Zanella-Cleon I, et al. Red blood cell generation from human induced pluripotent stem cells: perspectives for transfusion medicine. Haematologica. 2010; 95:1651–1659.
Article32. Peyrard T, Bardiaux L, Krause C, Kobari L, Lapillonne H, Andreu G, et al. Banking of pluripotent adult stem cells as an unlimited source for red blood cell production: potential applications for alloimmunized patients and rare blood challenges. Transfus Med Rev. 2011; 25:206–216.
Article33. Daley GQ. Stem cells: roadmap to the clinic. J Clin Invest. 2010; 120:8–10.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Production of Transfusable Red Blood Cells from Stem Cells
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Exogenous c-Myc Blocks Differentiation and Improves Expansion of Human Erythroblasts In vitro
- Genetically Engineered In Vitro Erythropoiesis
- Current status of red blood cell manufacturing in 3D culture and bioreactors