J Periodontal Implant Sci.
2017 Jun;47(3):143-153. 10.5051/jpis.2017.47.3.143.
Periodontal and endodontic pathology delays extraction socket healing in a canine model
- Affiliations
-
- 1Department of Periodontology and Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea. periokoo@snu.ac.kr
- 2US Army Advanced Education Program in Periodontics, Ft. Gordon, GA, USA.
- 3Department of Periodontology, Dankook University College of Dentistry, Cheonan, Korea.
- 4Department of Oral Surgery, Universitätsklinikum Düsseldorf, Düsseldorf, Germany.
- 5Laboratory for Applied Periodontal & Craniofacial Regeneration (LAPCR), Augusta University Dental College of Georgia, Augusta, GA, USA.
- KMID: 2383828
- DOI: http://doi.org/10.5051/jpis.2017.47.3.143
Abstract
- PURPOSE
The aim of the present exploratory study was to evaluate extraction socket healing at sites with a history of periodontal and endodontic pathology.
METHODS
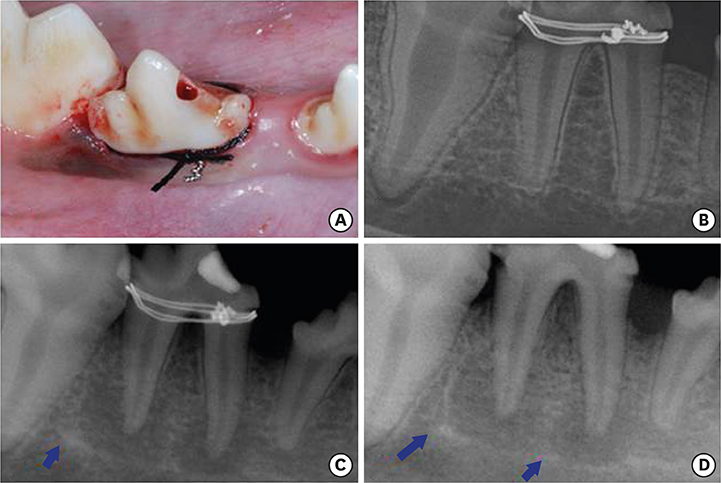
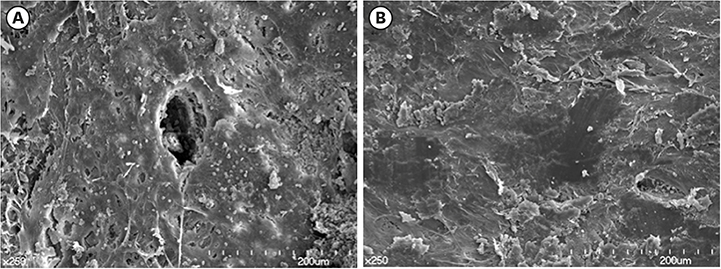
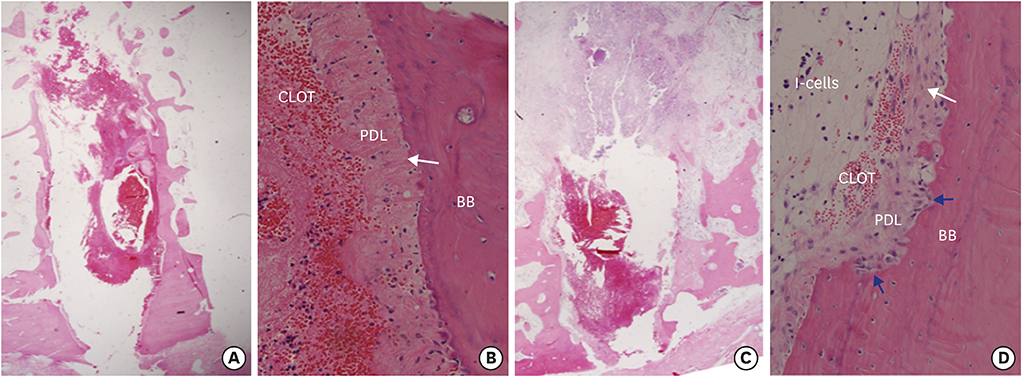
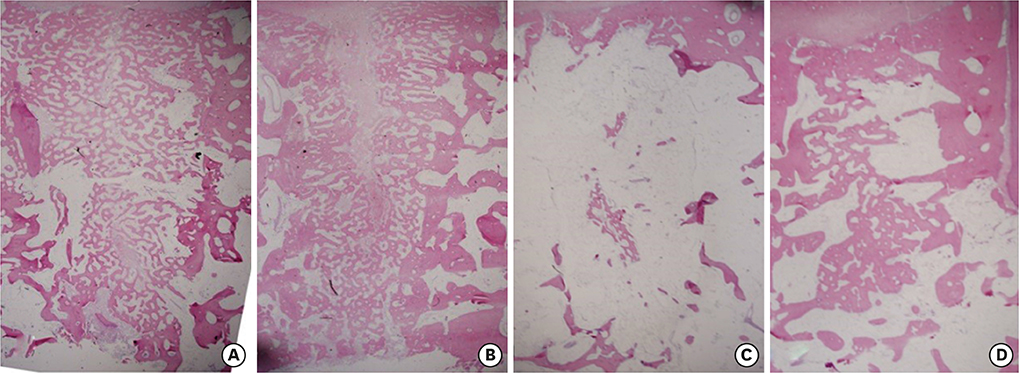
The mandibular 4th premolar teeth in 5 adult beagle dogs served as experimental units. Periodontal and endodontic lesions were induced in 1 premolar site in each animal using wire ligatures and pulpal exposure over 3 months (diseased sites). The contralateral premolar sites served as healthy controls. The mandibular 4th premolar teeth were then extracted with minimal trauma, followed by careful wound debridement. The animals were sacrificed at days 1, 7, 30, 60, and 90 post-extraction for analysis, and the healing patterns at the healthy and diseased extraction sites were compared using radiography, scanning electron microscopy, histology, and histometry.
RESULTS
During the first 7 days of healing, a significant presence of inflammatory granulation tissue was noted at the diseased sites (day 1), along with a slightly accelerated rate of fibrin clot resolution on day 7. On day 30, the diseased extraction sites showed a greater percentage of persistent fibrous connective tissue, and an absence of bone marrow formation. In contrast, healthy sites showed initial signs of bone marrow formation on day 30, and subsequently a significantly greater proportion of mature bone marrow formation on both days 60 and 90. Radiographs exhibited sclerotic changes adjoining apical endodontic lesions, with scanning electron microscopy showing collapsed Volkmann canals protruding from these regions in the diseased sites. Furthermore, periodontal ligament fibers exhibited a parallel orientation to the alveolar walls of the diseased sites, in contrast to a perpendicular arrangement in the healthy sites.
CONCLUSIONS
Within the limitations of this study, it appears that a history of periodontal and endodontic pathology may critically affect bone formation and maturation, leading to delayed and compromised extraction socket healing.
Keyword
MeSH Terms
Figure
Reference
-
1. Pietrokovski J, Massler M. Alveolar ridge resorption following tooth extraction. J Prosthet Dent. 1967; 17:21–27.
Article2. Amler MH. The time sequence of tissue regeneration in human extraction wounds. Oral Surg Oral Med Oral Pathol. 1969; 27:309–318.
Article3. Cardaropoli G, Araújo M, Lindhe J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J Clin Periodontol. 2003; 30:809–818.4. Schropp L, Kostopoulos L, Wenzel A. Bone healing following immediate versus delayed placement of titanium implants into extraction sockets: a prospective clinical study. Int J Oral Maxillofac Implants. 2003; 18:189–199.5. Araújo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol. 2005; 32:212–218.
Article6. Ahn JJ, Shin HI. Bone tissue formation in extraction sockets from sites with advanced periodontal disease: a histomorphometric study in humans. Int J Oral Maxillofac Implants. 2008; 23:1133–1138.7. Kim JH, Susin C, Min JH, Suh HY, Sang EJ, Ku Y, et al. Extraction sockets: erratic healing impeding factors. J Clin Periodontol. 2014; 41:80–85.
Article8. Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG; National Centre for the Replacement, Refinement and Reduction of Amimals in Research. Animal research: reporting in vivo experiments--the ARRIVE guidelines. J Cereb Blood Flow Metab. 2011; 31:991–993.
Article9. Buttke TM, Shipper G, Delano EO, Trope M. C-reactive protein and serum amyloid A in a canine model of chronic apical periodontitis. J Endod. 2005; 31:728–732.
Article10. Do MJ, Kim K, Lee H, Cha S, Seo T, Park HJ, et al. Development of animal experimental periodontitis models. J Periodontal Implant Sci. 2013; 43:147–152.
Article11. Cardaropoli G, Araújo M, Hayacibara R, Sukekava F, Lindhe J. Healing of extraction sockets and surgically produced - augmented and non-augmented - defects in the alveolar ridge. An experimental study in the dog. J Clin Periodontol. 2005; 32:435–440.
Article12. Araújo MG, Lindhe J. Ridge preservation with the use of Bio-Oss collagen: a 6-month study in the dog. Clin Oral Implants Res. 2009; 20:433–440.
Article13. Kim JJ, Song HY, Ben Amara H, Kyung-Rim K, Koo KT. Hyaluronic acid improves bone formation in extraction sockets with chronic pathology: a pilot study in dogs. J Periodontol. 2016; 87:790–795.
Article14. Lerut T, Nafteux P, Moons J, Coosemans W, Decker G, De Leyn P, et al. Three-field lymphadenectomy for carcinoma of the esophagus and gastroesophageal junction in 174 R0 resections: impact on staging, disease-free survival, and outcome: a plea for adaptation of TNM classification in upper-half esophageal carcinoma. Ann Surg. 2004; 240:962–972.15. Lin WL, McCulloch CA, Cho MI. Differentiation of periodontal ligament fibroblasts into osteoblasts during socket healing after tooth extraction in the rat. Anat Rec. 1994; 240:492–506.
Article16. Elfenbaum A. Condensing osteitis in the dental x-ray. Dent Dig. 1967; 73:554–558.17. Morse DR, Esposito JV, Yesilsoy C. Recall radiopaque response determined from radiographic examination of 211 consecutive cases with initial periapical pathosis. Quintessence Int. 1985; 16:419–428.18. Monahan R. Periapical and localized radiopacities. Dent Clin North Am. 1994; 38:113–136.19. Abrahams JJ, Berger SB. Inflammatory disease of the jaw: appearance on reformatted CT scans. AJR Am J Roentgenol. 1998; 170:1085–1091.
Article20. Nobuto T, Yanagihara K, Teranishi Y, Minamibayashi S, Imai H, Yamaoka A. Periosteal microvasculature in the dog alveolar process. J Periodontol. 1989; 60:709–715.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- EFFECT OF ELECTRICAL STIMULATION ON BONE FORMATION IN THE EXTRACTION SOCKET OF RAT
- Effect of immediate provisional restoration on the preservation of gingival contour
- Dry Socket Etiology, Diagnosis, and Clinical Treatment Techniques
- Cases report of autotransplantation of immature teeth
- Clinical, radiologic, and histopathologic analysis of diseases developed in delayed wound healing of extraction socket