Korean J Gastroenterol.
2017 Apr;69(4):226-231. 10.4166/kjg.2017.69.4.226.
Fecal Microbiota Transplantation for Refractory and Recurrent Clostridium difficile Infection: A Case Series of Nine Patients
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, Inha University School of Medicine, Incheon, Korea. ywshin@inha.ac.kr
- 2Division of Infectious Diseases, Department of Internal Medicine, Inha University School of Medicine, Incheon, Korea.
- KMID: 2383400
- DOI: http://doi.org/10.4166/kjg.2017.69.4.226
Abstract
- BACKGROUND/AIMS
Fecal microbiota transplantation (FMT) is a highly effective therapy for refractory and recurrent Clostridium difficile infection (CDI). Despite its excellent efficacy and recent widespread use, FMT has not been widely used in South Korea thus far. We describe our experience with FMT to treat refractory/recurrent CDI.
METHODS
We conducted a chart review of patients who underwent FMT for refractory/recurrent CDI at Inha University Hospital, between March 2014 and June 2016. The demographic information, treatment data, and adverse events were reviewed. FMT was administered via colonoscopy and/or duodenoscopy. All stool donors were rigorously screened to prevent infectious disease transmission.
RESULTS
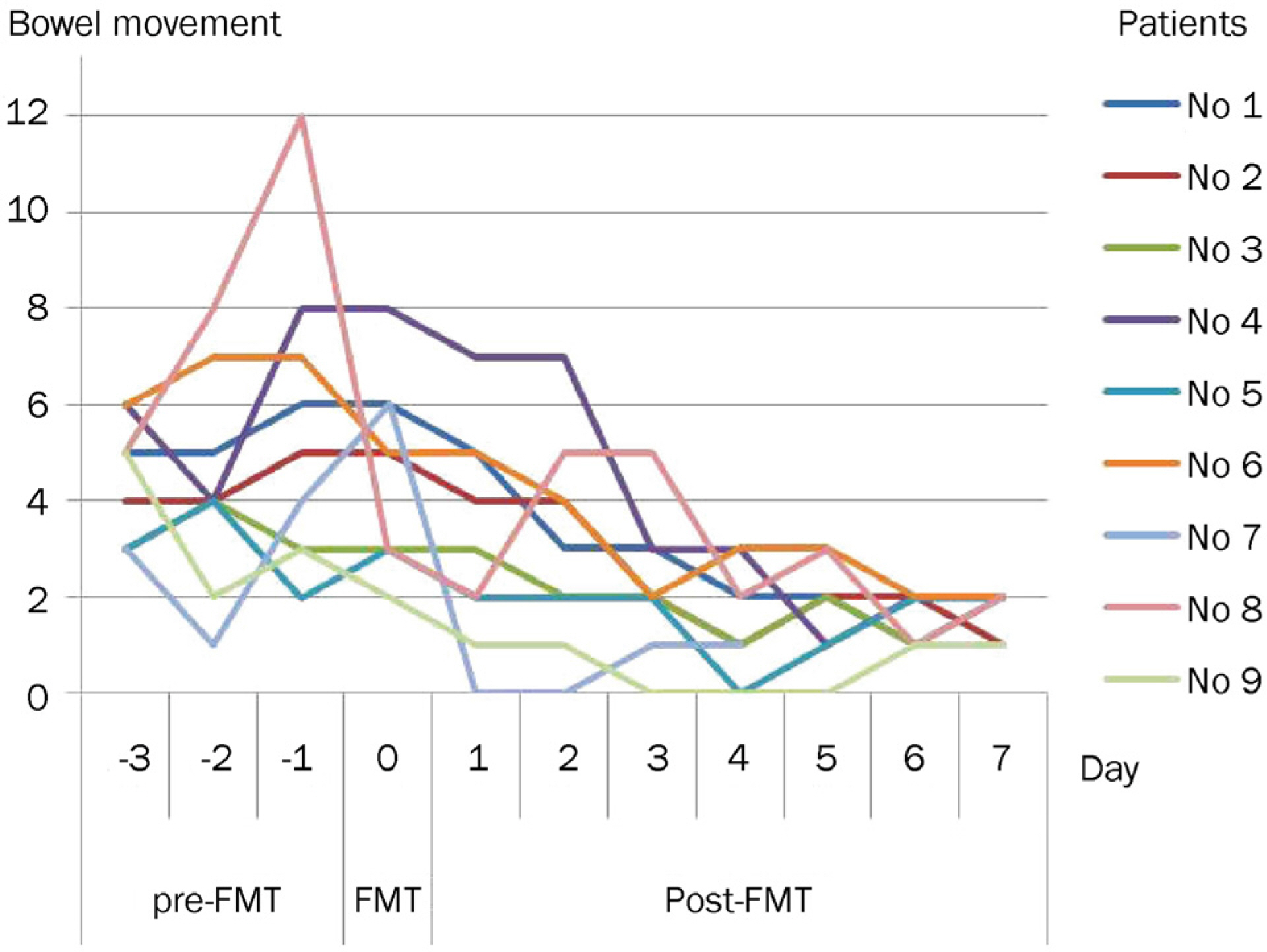
FMT was performed in nine patients with refractory/recurrent CDI. All patients were dramatically cured. Bowel movement was normalized within one week after FMT. There were no procedure-related adverse events, except aspiration pneumonia in one patient. During the follow-up period (mean 11.4 months), recurrence of CDI was observed in one patient at one month after FMT due to antibiotics.
CONCLUSIONS
FMT is a safe, well-tolerated and highly effective treatment for refractory/recurrent CDI. Although there are many barriers to using FMT, we expect that FMT will be widely used to treat refractory/recurrent CDI in South Korea.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Freeman J, Bauer MP, Baines SD, et al. The changing epidemiology of clostridium difficile infections. Clin Microbiol Rev. 2010; 23:529–549.2. McFarland LV, Surawicz CM, Rubin M, Fekety R, Elmer GW, Greenberg RN. Recurrent clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol. 1999; 20:43–50.
Article3. Lee CH, Belanger JE, Kassam Z, et al. The outcome and long-term follow-up of 94 patients with recurrent and refractory clostridium difficile infection using single to multiple fecal microbiota transplantation via retention enema. Eur J Clin Microbiol Infect Dis. 2014; 33:1425–1428.
Article4. Rossen NG, MacDonald JK, de Vries EM, et al. Fecal microbiota transplantation as novel therapy in gastroenterology: a systematic review. World J Gastroenterol. 2015; 21:5359–5371.
Article5. Kelly CR, Kahn S, Kashyap P, et al. Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology. 2015; 149:223–237.
Article6. Weingarden A, González A, Vázquez-Baeza Y, et al. Dynamic changes in short- and long-term bacterial composition following fecal microbiota transplantation for recurrent clostridium difficile infection. Microbiome. 2015; 3:10.
Article7. Debast SB, Bauer MP, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases. European society of clinical microbiology and infectious diseases: update of the treatment guidance document for clostridium difficile infection. Clin Microbiol Infect. 2014; 20(Suppl 2):1–26.
Article8. Gweon TG, Lee KJ, Kang DH, et al. A case of toxic megacolon caused by clostridium difficile infection and treated with fecal microbiota transplantation. Gut Liver. 2015; 9:247–250.
Article9. Shin JY, Ko EJ, Lee SH, et al. Refractory pseudomembranous colitis that was treated successfully with colonoscopic fecal microbial transplantation. Intest Res. 2016; 14:83–88.
Article10. Jeon YD, Hong N, Kim JH, et al. Fecal transplantation using a na-soenteric tube during an initial episode of severe clostridium difficile infection. Infect Chemother. 2016; 48:31–35.
Article11. Gweon TG, Kim J, Lim CH, et al. Fecal microbiota transplantation using upper gastrointestinal tract for the treatment of refractory or severe complicated clostridium difficile infection in elderly patients in poor medical condition: the first study in an Asian country. Gastroenterol Res Pract. 2016; 2016:2687605.12. Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014; 109:1065–1071.
Article13. Palmer R. Fecal matters. Nat Med. 2011; 17:150–152.
Article14. Zipursky JS, Sidorsky TI, Freedman CA, Sidorsky MN, Kirkland KB. Physician attitudes toward the use of fecal microbiota transplantation for the treatment of recurrent clostridium difficile infection. Can J Gastroenterol Hepatol. 2014; 28:319–324.15. Lessa FC, Mu Y, Bamberg WM, et al. Burden of clostridium difficile infection in the United States. N Engl J Med. 2015; 372:825–834.
Article16. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of clostridium difficile infections. Am J Gastroenterol. 2013; 108:478–498. quiz 499.
Article17. Garey KW, Sethi S, Yadav Y, DuPont HL. Meta-analysis to assess risk factors for recurrent clostridium difficile infection. J Hosp Infect. 2008; 70:298–304.18. Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing clostridium difficile infection. JAMA. 2014; 312:1772–1778.19. Tian H, Ding C, Gong J, Wei Y, McFarland LV, Li N. Freeze-dried, capsulized fecal microbiota transplantation for relapsing clostridium difficile infection. J Clin Gastroenterol. 2015; 49:537–538.
Article20. Petrof EO, Gloor GB, Vanner SJ, et al. Stool substitute transplant therapy for the eradication of clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome. 2013; 1:3.
Article21. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011; 364:422–431.22. Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015; 372:1539–1548.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Refractory Clostridium difficile Infection Cured With Fecal Microbiota Transplantation in Vancomycin-Resistant Enterococcus Colonized Patient
- A Case of Toxic Megacolon Caused by Clostridium difficile Infection and Treated with Fecal Microbiota Transplantation
- Fecal Microbiota Transplantation as a Treatment of Recurrent Clostridium difficile Infection: Where Are We Now and Where Are We Heading?
- Two Cases of Refractory Pseudomembranous Colitis that Healed Following Fecal Microbiota Transplantation
- Successful Fecal Transplantation by Enema for Recurrent and Refractory Clostridium difficile Infection