Lab Med Online.
2017 Jul;7(3):103-110. 10.3343/lmo.2017.7.3.103.
Frequency and Distinct Characteristics of Acute Myeloid Leukemia Lacking HLA-DR and CD34 Expression: Features Intermediate between Typical Acute Myeloid Leukemia and Acute Promyelocytic Leukemia
- Affiliations
-
- 1Department of Laboratory Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea. yucho@amc.seoul.kr
- KMID: 2383088
- DOI: http://doi.org/10.3343/lmo.2017.7.3.103
Abstract
- BACKGROUND
The objective of this study was to investigate the frequency and characteristics of HLA-DRâ»/CD34â» acute myeloid leukemia (AML) also known as acute promyelocytic leukemia (APL)-like AML.
METHODS
This study included 683 newly diagnosed patients with AML. After exclusion of 211 patients with recurrent genetic abnormalities, one with acute panmyelosis with myelofibrosis, two with myeloid leukemia associated with Down syndrome, and two devoid of metaphase cells, we classified the remaining 467 patients as follows: group 1, HLA-DRâº/CD34⺠(typical AML); group 2, HLA-DRâº/CD34â» or HLA-DRâ»/CD34âº; group 3, APL-like AML.
RESULTS
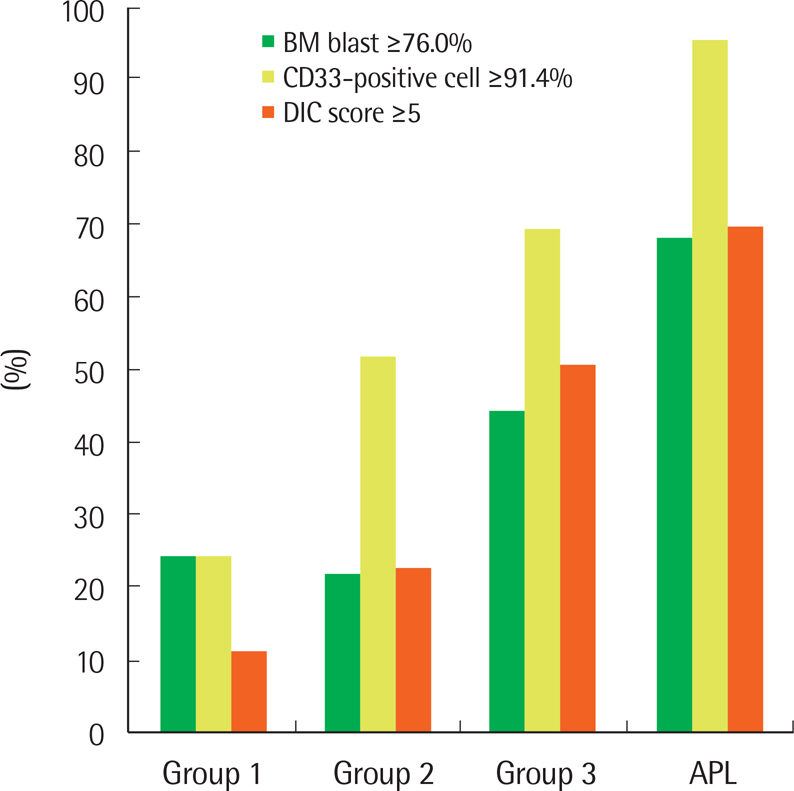
Group 1 comprised 294 patients, group 2 comprised 133, and group 3 comprised 40. Therefore, the frequency of APL-like AML among 683 unselected patients with AML was 5.9%. Group 3 patients had significantly higher leukocyte counts and bone marrow (BM) blast percentages, higher frequencies of normal karyotypes and NPM1 mutation, higher fractions of CD33-positive cells, higher concentrations of fibrin degradation products and D-dimers, lower frequencies of complex karyotypes, monosomal karyotypes and poor cytogenetic risk, lower fractions of CD13-positive cells, and lower fibrinogen concentrations, compared with group 1 patients. The values of the BM blast percentage, number of CD33-positive cells, and DIC score of the patients with APL-like AML were intermediate between those of the patients with typical AML and APL.
CONCLUSIONS
This study demonstrates that APL-like AML is not uncommon, and it has characteristics distinguishable from those of typical AML. APL-like AML may have some pathophysiological relationships with APL, which need further investigation.
Keyword
MeSH Terms
-
Bone Marrow
Cytogenetics
Dacarbazine
Down Syndrome
Fibrin Fibrinogen Degradation Products
Fibrinogen
HLA-DR Antigens*
Humans
Karyotype
Leukemia, Myeloid
Leukemia, Myeloid, Acute*
Leukemia, Promyelocytic, Acute*
Leukocyte Count
Metaphase
Primary Myelofibrosis
Dacarbazine
Fibrin Fibrinogen Degradation Products
Fibrinogen
HLA-DR Antigens
Figure
Reference
-
1. Arber DA, Vardiman JW, Brunning RD, Porwit A, Le Beau MM, Thiele J, et al. Acute myeloid leukaemia with recurrent genetic abnormalites. Swerdlow SH, Campo E, editors. eds.WHO classifcation of tumours of haematopoietic and lymphoid tissues. 4th ed.Lyon: IARC;2008. p. 110–23.2. Park JH, Qiao B, Panageas KS, Schymura MJ, Jurcic JG, Rosenblat TL, et al. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood. 2011; 118:1248–54.
Article3. Wetzler M, McElwain BK, Stewart CC, Blumenson L, Mortazavi A, Ford LA, et al. HLA-DR antigen-negative acute myeloid leukemia. Leukemia. 2003; 17:707–15.
Article4. Moon H, Lee S, Huh J, Chung WS. Characteristics of acute myeloid leukemia without HLA-DR expression. Korean J Lab Med. 2007; 27:313–7.
Article5. Syampurnawati M, Tatsumi E, Furuta K, Takenokuchi M, Nakamachi Y, Kawano S, et al. HLA-DR-negative AML (M1 and M2): FLT3 mutations (ITD and D835) and cell-surface antigen expression. Leuk Res. 2007; 31:921–9.
Article6. Syampurnawati M, Tatsumi E, Ardianto B, Takenokuchi M, Nakamachi Y, Kawano S, et al. DR negativity is a distinctive feature of M1/M2 AML cases with NPM1 mutation. Leuk Res. 2008; 32:1141–3.
Article7. Oelschlaegel U, Mohr B, Schaich M, Schakel U, Kroschinsky F, Illmer T, et al. HLA-DRneg patients without acute promyelocytic leukemia show distinct immunophenotypic, genetic, molecular, and cytomor-phologic characteristics compared to acute promyelocytic leukemia. Cytometry B Clin Cytom. 2009; 76:321–7.8. Ferrari A, Bussaglia E, Ubeda J, Facchini L, Aventin A, Sierra J, et al. Immunophenotype distinction between acute promyelocytic leukaemia and CD15- CD34- HLA-DR- acute myeloid leukaemia with nucleophosmin mutations. Hematol Oncol. 2012; 30:109–14.
Article9. Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the frst cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2011; 98:1752–9.10. Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005; 352:254–66.
Article11. Wouters BJ, Löwenberg B, Erpelinck-Verschueren CA, van Putten WL, Valk PJ, Delwel R. Double CEBPA mutations, but not single CEBPA mutations, defne a subgroup of acute myeloid leukemia with a distinctive gene expression profle that is uniquely associated with a favorable outcome. Blood. 2009; 113:3088–91.12. Schnittger S, Kinkelin U, Schoch C, Heinecke A, Haase D, Haferlach T, et al. Screening for MLL tandem duplication in 387 unselected patients with AML identify a prognostically unfavorable subset of AML. Leukemia. 2000; 14:796–804.
Article13. NCCN, NCCN Clinical Practice Guidelines in Oncology: Acute Myeloid Leukemia. Version 2. 2016.https://www.nccn.org/professionals/physi-cian_gls/pdf/aml.pdf.14. Breems DA, Van Putten WL, De Greef GE, Van Zelderen-Bhola SL, Ger-ssen-Schoorl KB, Mellink CH, et al. Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. J Clin Oncol. 2008; 26:4791–7.
Article15. Taylor FB Jr, Toh CH, Hoots WK, Wada H, Levi M. Scientifc Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards defnition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001; 86:1327–30.16. Gorczyca W, Sun ZY, Cronin W, Li X, Mau S, Tugulea S. Immunophenotypic pattern of myeloid populations by fow cytometry analysis. Methods Cell Biol. 2011; 103:221–66.17. Scott AA, Head DR, Kopecky KJ, Appelbaum FR, Theil KS, Grever MR, et al. HLA-DR-, CD33+, CD56+, CD16- myeloid/natural killer cell acute leukemia: a previously unrecognized form of acute leukemia poten-tially misdiagnosed as French-American-British acute myeloid leukemia-M3. Blood. 1994; 84:244–55.18. Alcalay M, Tiacci E, Bergomas R, Bigerna B, Venturini E, Minardi SP, et al. Acute myeloid leukemia bearing cytoplasmic nucleophosmin (NPMc+ AML) shows a distinct gene expression profle characterized by up-regulation of genes involved in stem-cell maintenance. Blood. 2005; 106:899–902.19. Haferlach C, Mecucci C, Schnittger S, Kohlmann A, Mancini M, Cuneo A, et al. AML with mutated NPM1 carrying a normal or aberrant karyotype show overlapping biologic, pathologic, immunophenotypic, and prognostic features. Blood. 2009; 114:3024–32.
Article20. Schlenk RF, Döhner K, Kneba M, Götze K, Hartmann F, Del Valle F, et al. Gene mutations and response to treatment with all-trans retinoic acid in elderly patients with acute myeloid leukemia. Results from the AMLSG Trial AML HD98B. Haematologica. 2009; 94:54–60.
Article21. Martelli MP, Gionfriddo I, Mezzasoma F, Milano F, Pierangeli S, Mulas F, et al. Arsenic trioxide and all-trans retinoic acid target NPM1 mutant oncoprotein levels and induce apoptosis in NPM1-mutated AML cells. Blood. 2015; 125:3455–65.
Article22. de The H, Le Bras M, Lallemand-Breitenbach V. The cell biology of disease: Acute promyelocytic leukemia, arsenic, and PML bodies. J Cell Biol. 2012; 198:11–21.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute Myeloid Leukemia with Intracardiac Thrombus Presenting as Acute Limb Ischemia
- A Case of Acute Myeloid Leukemia with an Unusual Phenotype
- Characteristics of Acute Myeloid Leukemia without HLA-DR Expression
- A Case of Acute Promyelocytic Leukemia with Co-existence of BCR-ABL1 and PML-RARA Rearrangements Detected by PCR
- Acute myeloid leukemia arising from chronic myelomonocytic leukemia during hypomethylating therapy