Ann Rehabil Med.
2016 Oct;40(5):757-768. 10.5535/arm.2016.40.5.757.
A Model of Glial Scarring Analogous to the Environment of a Traumatically Injured Spinal Cord Using Kainate
- Affiliations
-
- 1Department of Rehabilitation Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 2Department of Physical Medicine and Rehabilitation, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea. chhwang1220ciba@gmail.com
- 3Department of Anatomy, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2382907
- DOI: http://doi.org/10.5535/arm.2016.40.5.757
Abstract
OBJECTIVE
To develop an in vitro model analogous to the environment of traumatic spinal cord injury (SCI), the authors evaluated change of astrogliosis following treatments with kainate and/or scratch, and degree of neurite outgrowth after treatment with a kainate inhibitor.
METHODS
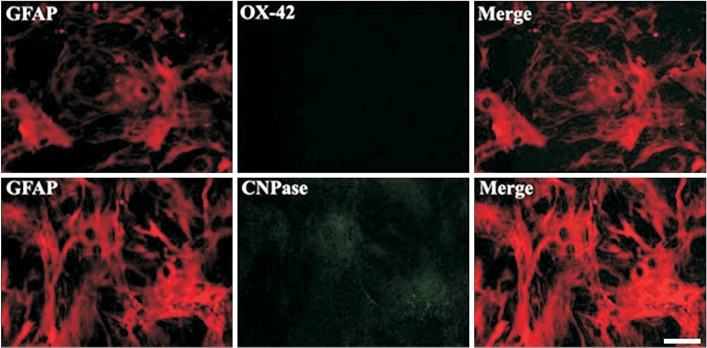
Astrocytes were obtained from the rat spinal cord. Then, 99% of the cells were confirmed to be GFAP-positive astrocytes. For chemical injury, the cells were treated with kainate at different concentrations (10, 50 or 100 µM). For mechanical injury, two kinds of uniform scratches were made using a plastic pipette tip by removing strips of cells. For combined injury (S/K), scratch and kainate were provided. Cord neurons from rat embryos were plated onto culture plates immediately after the three kinds of injuries and some cultures were treated with a kainate inhibitor.
RESULTS
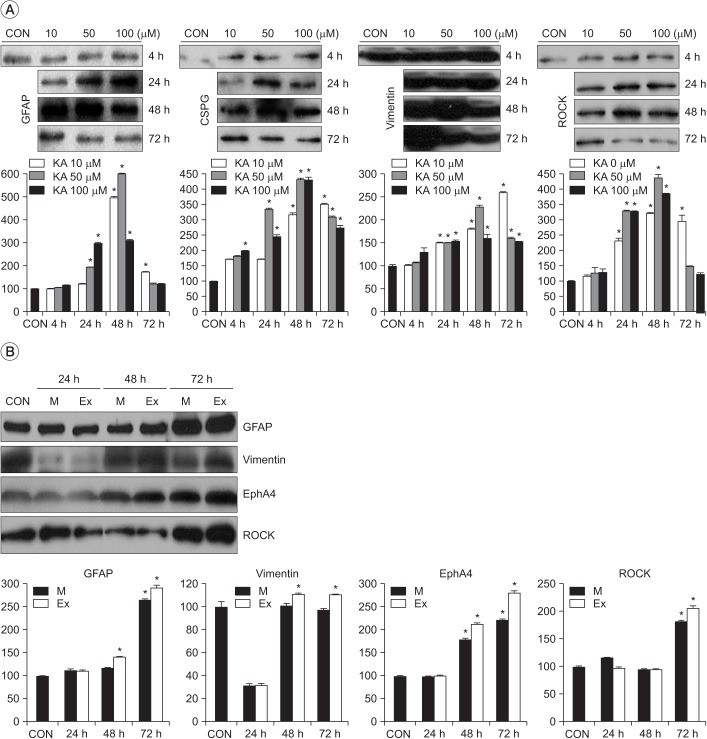
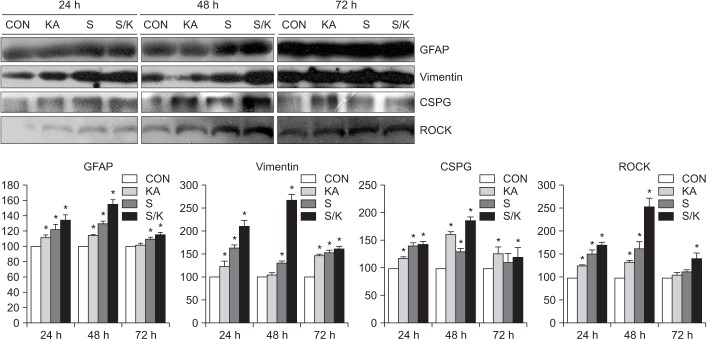
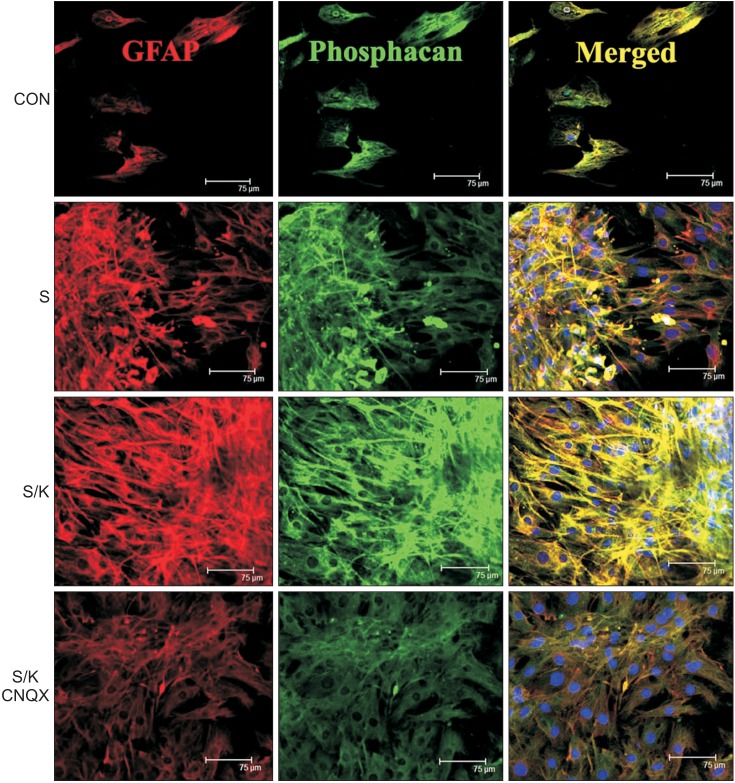
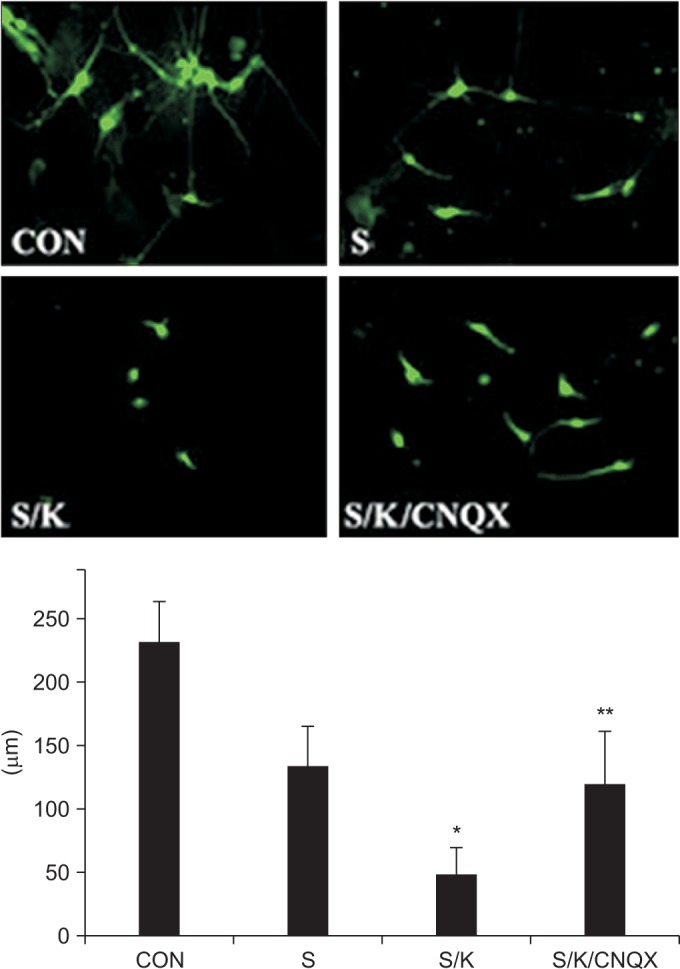
Astro-gliosis (glial fibrillary acidic protein [GFAP], vimentin, chondroitin sulfate proteoglycan [CSPG], rho-associated protein kinase [ROCK], and ephrin type-A receptor 4 [EphA4]) was most prominent after treatment with 50 µM kainate and extensive scratch injury in terms of single arm (p<0.001) and in the S/K-induced injury model in view of single or combination (p<0.001). Neurite outgrowth in the seeded spinal cord (β-III tubulin) was the least in the S/K-induced injury model (p<0.001) and this inhibition was reversed by the kainate inhibitor (p<0.001).
CONCLUSION
The current in vitro model combining scratch and kainate induced glial scarring and inhibitory molecules and restricted neurite outgrowth very strongly than either the mechanically or chemically-induced injury model; hence, it may be a useful tool for research on SCI.
MeSH Terms
Figure
Cited by 1 articles
-
Role of Agmatine on Neuroglia in Central Nervous System Injury
Sumit Barua, Jong Youl Kim, Jong Eun Lee
Brain Neurorehabil. 2019;12(1):. doi: 10.12786/bn.2019.12.e2.
Reference
-
1. Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004; 5:146–156. PMID: 14735117.
Article2. McKeon RJ, Jurynec MJ, Buck CR. The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J Neurosci. 1999; 19:10778–10788. PMID: 10594061.
Article3. Hatten ME, Liem RK, Shelanski ML, Mason CA. Astroglia in CNS injury. Glia. 1991; 4:233–243. PMID: 1827781.
Article4. Yu AC, Lee YL, Eng LF. Astrogliosis in culture. I: The model and the effect of antisense oligonucleotides on glial fibrillary acidic protein synthesis. J Neurosci Res. 1993; 34:295–303. PMID: 8455207.
Article5. Calvo JL, Carbonell AL, Boya J. Co-expression of glial fibrillary acidic protein and vimentin in reactive astrocytes following brain injury in rats. Brain Res. 1991; 566:333–336. PMID: 1814551.6. Fronza M, Heinzmann B, Hamburger M, Laufer S, Merfort I. Determination of the wound healing effect of Calendula extracts using the scratch assay with 3T3 fibroblasts. J Ethnopharmacol. 2009; 126:463–467. PMID: 19781615.
Article7. Nishio T, Kawaguchi S, Yamamoto M, Iseda T, Kawasaki T, Hase T. Tenascin-C regulates proliferation and migration of cultured astrocytes in a scratch wound assay. Neuroscience. 2005; 132:87–102. PMID: 15780469.
Article8. Smith AN, Willis E, Chan VT, Muffley LA, Isik FF, Gibran NS, et al. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp Cell Res. 2010; 316:48–54. PMID: 19666021.
Article9. Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004; 21:754–774. PMID: 15253803.
Article10. Matute C, Domercq M, Sanchez-Gomez MV. Glutamate-mediated glial injury: mechanisms and clinical importance. Glia. 2006; 53:212–224. PMID: 16206168.
Article11. Liu D, Xu GY, Pan E, McAdoo DJ. Neurotoxicity of glutamate at the concentration released upon spinal cord injury. Neuroscience. 1999; 93:1383–1389. PMID: 10501463.
Article12. Olney JW, Rhee V, Ho OL. Kainic acid: a powerful neurotoxic analogue of glutamate. Brain Res. 1974; 77:507–512. PMID: 4152936.
Article13. Mazzone GL, Margaryan G, Kuzhandaivel A, Nasrabady SE, Mladinic M, Nistri A. Kainate-induced delayed onset of excitotoxicity with functional loss unrelated to the extent of neuronal damage in the in vitro spinal cord. Neuroscience. 2010; 168:451–462. PMID: 20362644.
Article14. Yang H, Cheng XP, Li JW, Yao Q, Ju G. De-differentiation response of cultured astrocytes to injury induced by scratch or conditioned culture medium of scratch-insulted astrocytes. Cell Mol Neurobiol. 2009; 29:455–473. PMID: 19130217.
Article15. Yoshimura E, Majima A, Sakakura Y, Sakakura T, Yoshida T. Expression of tenascin-C and the integrin alpha 9 subunit in regeneration of rat nasal mucosa after chemical injury: involvement in migration and proliferation of epithelial cells. Histochem Cell Biol. 1999; 111:259–264. PMID: 10219625.16. O'Callaghan JP, Jensen KF, Miller DB. Quantitative aspects of drug and toxicant-induced astrogliosis. Neurochem Int. 1995; 26:115–124. PMID: 7599532.17. Ellis EF, McKinney JS, Willoughby KA, Liang S, Povlishock JT. A new model for rapid stretch-induced injury of cells in culture: characterization of the model using astrocytes. J Neurotrauma. 1995; 12:325–339. PMID: 7473807.
Article18. Milenkovic I, Nedeljkovic N, Filipovic R, Pekovic S, Culic M, Rakic L, et al. Pattern of glial fibrillary acidic protein expression following kainate-induced cerebellar lesion in rats. Neurochem Res. 2005; 30:207–213. PMID: 15895824.
Article19. David JC, Yamada KA, Bagwe MR, Goldberg MP. AMPA receptor activation is rapidly toxic to cortical astrocytes when desensitization is blocked. J Neurosci. 1996; 16:200–209. PMID: 8613786.
Article20. Malhotra SK, Luong LT, Bhatnagar R, Shnitka TK. Up-regulation of reactive astrogliosis in the rat glioma 9L cell line by combined mechanical and chemical injuries. Cytobios. 1997; 89:115–134. PMID: 9363621.21. Wanner IB, Deik A, Torres M, Rosendahl A, Neary JT, Lemmon VP, et al. A new in vitro model of the glial scar inhibits axon growth. Glia. 2008; 56:1691–1709. PMID: 18618667.22. Gaviria M, Privat A, d'Arbigny P, Kamenka JM, Haton H, Ohanna F. Neuroprotective effects of gacyclidine after experimental photochemical spinal cord lesion in adult rats: dose-window and time-window effects. J Neurotrauma. 2000; 17:19–30. PMID: 10674755.
Article23. Rosenberg LJ, Teng YD, Wrathall JR. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline reduces glial loss and acute white matter pathology after experimental spinal cord contusion. J Neurosci. 1999; 19:464–475. PMID: 9870974.
Article24. Wrathall JR, Teng YD, Choiniere D. Amelioration of functional deficits from spinal cord trauma with systemically administered NBQX, an antagonist of non-N-methyl-D-aspartate receptors. Exp Neurol. 1996; 137:119–126. PMID: 8566203.
Article25. Chang ML, Wu CH, Jiang-Shieh YF, Shieh JY, Wen CY. Reactive changes of retinal astrocytes and Müller glial cells in kainate-induced neuroexcitotoxicity. J Anat. 2007; 210:54–65. PMID: 17229283.
Article26. Kohno H, Sakai T, Kitahara K. Induction of nestin, Ki-67, and cyclin D1 expression in Müller cells after laser injury in adult rat retina. Graefes Arch Clin Exp Ophthalmol. 2006; 244:90–95. PMID: 15983812.
Article27. Schnell L, Fearn S, Klassen H, Schwab ME, Perry VH. Acute inflammatory responses to mechanical lesions in the CNS: differences between brain and spinal cord. Eur J Neurosci. 1999; 11:3648–3658. PMID: 10564372.
Article28. McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991; 11:3398–3411. PMID: 1719160.
Article29. Maxwell WL, Follows R, Ashhurst DE, Berry M. The response of the cerebral hemisphere of the rat to injury. II: The neonatal rat. Philos Trans R Soc Lond B Biol Sci. 1990; 328:501–513. PMID: 1974075.30. Shearer MC, Niclou SP, Brown D, Asher RA, Holtmaat AJ, Levine JM, et al. The astrocyte/meningeal cell interface is a barrier to neurite outgrowth which can be overcome by manipulation of inhibitory molecules or axonal signalling pathways. Mol Cell Neurosci. 2003; 24:913–925. PMID: 14697658.
Article31. Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988; 1:623–634. PMID: 2908446.
Article32. Akins PT, Atkinson RP. Glutamate AMPA receptor antagonist treatment for ischaemic stroke. Curr Med Res Opin. 2002; 18(Suppl 2):s9–s13. PMID: 12365832.
Article33. Lerma J. Roles and rules of kainate receptors in synaptic transmission. Nat Rev Neurosci. 2003; 4:481–495. PMID: 12778120.
Article34. Jones LL, Sajed D, Tuszynski MH. Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition. J Neurosci. 2003; 23:9276–9288. PMID: 14561854.
Article35. Hansson E, Muyderman H, Leonova J, Allansson L, Sinclair J, Blomstrand F, et al. Astroglia and glutamate in physiology and pathology: aspects on glutamate transport, glutamate-induced cell swelling and gap-junction communication. Neurochem Int. 2000; 37:317–329. PMID: 10812217.
Article36. Gottlieb M, Matute C. Expression of ionotropic glutamate receptor subunits in glial cells of the hippocampal CA1 area following transient forebrain ischemia. J Cereb Blood Flow Metab. 1997; 17:290–300. PMID: 9119902.
Article37. Simantov R, Crispino M, Hoe W, Broutman G, Tocco G, Rothstein JD, et al. Changes in expression of neuronal and glial glutamate transporters in rat hippocampus following kainate-induced seizure activity. Brain Res Mol Brain Res. 1999; 65:112–123. PMID: 10036313.
Article38. Aono S, Kashiwamata S. GFAP under physiological and pathological conditions. Med Sci Res. 1990; 18:235–239.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Fetal Spinal Cord Transplants on Injured Rat Spinal Cord
- Fracture of Femur Neck with Heterotopic Ossification in Spinal Cord Injured Patient
- Olig2-expressing Mesenchymal Stem Cells Enhance Functional Recovery after Contusive Spinal Cord Injury
- Activation of Embryonic Intermediate Filaments Contributes to Glial Scar Formation after Spinal Cord Injury in Rats
- The Influence of Self-care Agency and Social Support on Self-care Practice among Spinal Cord Injured Patients