J Adv Prosthodont.
2017 Jun;9(3):182-187. 10.4047/jap.2017.9.3.182.
The effect of prolonged storage and disinfection on the dimensional stability of 5 vinyl polyether silicone impression materials
- Affiliations
-
- 1Faculty of Medicine and Dentistry, Department of Dentistry, University of Alberta, Edmonton, Alberta, Canada. unassar@ualberta.ca
- KMID: 2382604
- DOI: http://doi.org/10.4047/jap.2017.9.3.182
Abstract
- PURPOSE
Vinyl polyether silicone (VPES) has a different composition from other elastomeric impression materials as it combines vinyl polysiloxane (VPS) and polyether (PE). Therefore, it is important to study its properties and behavior under different test conditions. This study investigated the dimensional stability of 5 VPES consistencies when stored for up to 2 weeks, with and without using a standard disinfection procedure.
MATERIALS AND METHODS
40 discs of each VPES consistency (total 200) were made using a stainless steel die and ring as described by ANSI /ADA specification No. 19. 20 discs of each material were immersed in a 2.5% buffered glutaraldehyde solution for 30 minutes. Dimensional stability measurements were calculated immediately after fabrication and repeated on the same discs after 7 and 14 days of storage. The data was analyzed using two-way ANOVA with a significance level set at α = 0.05.
RESULTS
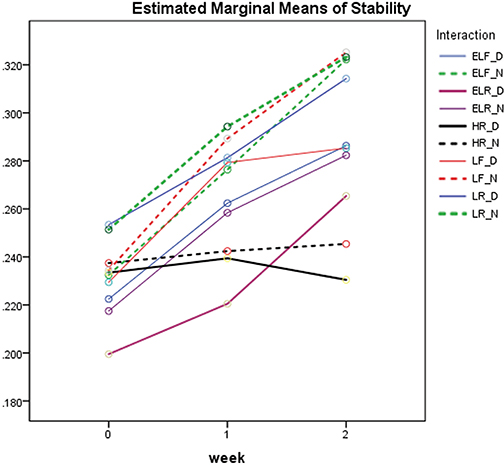
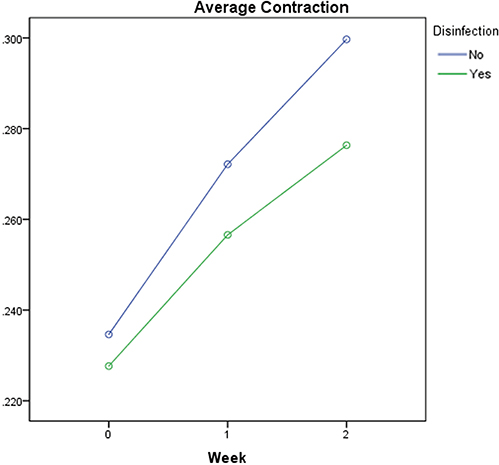
The discs mean contraction was below 0.5% at all test times ranging from 0.200 ± 0.014 to 0.325 ± 0.007. Repeated measures ANOVA showed a statistically significant difference after 2-week storage between the disinfected and non-disinfected groups (P < .001). Although there was no statistically significant difference between the materials at the time of fabrication, the contraction of the materials increased with storage for 1 and 2 weeks.
CONCLUSION
The dimensional changes of VPES impression discs after disinfection and prolonged storage complied with ANSI/ADA standard. The tested VPES impression materials were dimensionally stable for clinical use after disinfection for 30 minutes in glutaraldehyde and storage for up to 2 weeks.
MeSH Terms
Figure
Reference
-
1. Sakaguchi RL, Powers JM. Craig's restorative dental materials. 13th ed. Philadelphia: Mosby Elsevier;2012. p. 293.2. Kanehira M, Finger WJ, Endo T. Volatilization of components from and water absorption of polyether impressions. J Dent. 2006; 34:134–138.3. Walker MP, Petrie CS, Haj-Ali R, Spencer P, Dumas C, Williams K. Moisture effect on polyether and polyvinylsiloxane dimensional accuracy and detail reproduction. J Prosthodont. 2005; 14:158–163.4. Petrie CS, Walker MP, O'mahony AM, Spencer P. Dimensional accuracy and surface detail reproduction of two hydrophilic vinyl polysiloxane impression materials tested under dry, moist, and wet conditions. J Prosthet Dent. 2003; 90:365–372.5. Corso M, Abanomy A, Di Canzio J, Zurakowski D, Morgano SM. The effect of temperature changes on the dimensional stability of polyvinyl siloxane and polyether impression materials. J Prosthet Dent. 1998; 79:626–631.6. Yilmaz H, Aydin C, Gul B, Yilmaz C, Semiz M. Effect of disinfection on the dimensional stability of polyether impression materials. J Prosthodont. 2007; 16:473–479.7. Kern M, Rathmer RM, Strub JR. Three-dimensional investigation of the accuracy of impression materials after disinfection. J Prosthet Dent. 1993; 70:449–456.8. Jagger DC, Vowles RW, McNally L, Davis F, O'Sullivan DJ. The effect of a range of disinfectants on the dimensional accuracy and stability of some impression materials. Eur J Prosthodont Restor Dent. 2007; 15:23–28.9. Melilli D, Rallo A, Cassaro A, Pizzo G. The effect of immersion disinfection procedures on dimensional stability of two elastomeric impression materials. J Oral Sci. 2008; 50:441–446.10. Wadhwani CP, Johnson GH, Lepe X, Raigrodski AJ. ccuracy of newly formulated fast-setting elastomeric impression materials. J Prosthet Dent. 2005; 93:530–539.11. Adabo GL, Zanarotti E, Fonseca RG, Cruz CA. Effect of disinfectant agents on dimensional stability of elastomeric impression materials. J Prosthet Dent. 1999; 81:621–624.12. Davis BA, Powers JM. Effect of immersion disinfection on properties of impression materials. J Prosthodont. 1994; 3:31–34.13. Rios MP, Morgano SM, Stein RS, Rose L. Effects of chemical disinfectant solutions on the stability and accuracy of the dental impression complex. J Prosthet Dent. 1996; 76:356–362.14. Lepe X, Johnson GH, Berg JC, Aw TC, Stroh GS. Wettability, imbibition, and mass change of disinfected low-viscosity impression materials. J Prosthet Dent. 2002; 88:268–276.15. Langenwalter EM, Aquilino SA, Turner KA. The dimensional stability of elastomeric impression materials following disinfection. J Prosthet Dent. 1990; 63:270–276.16. Matyas J, Dao N, Caputo AA, Lucatorto FM. Effects of disinfectants on dimensional accuracy of impression materials. J Prosthet Dent. 1990; 64:25–31.17. Thouati A, Deveaux E, Iost A, Behin P. Dimensional stability of seven elastomeric impression materials immersed in disinfectants. J Prosthet Dent. 1996; 76:8–14.18. Lepe X, Johnson GH. Accuracy of polyether and addition silicone after long-term immersion disinfection. J Prosthet Dent. 1997; 78:245–249.19. Walker MP, Rondeau M, Petrie C, Tasca A, Williams K. Surface quality and long-term dimensional stability of current elastomeric impression materials after disinfection. J Prosthodont. 2007; 16:343–351.20. Donovan TE, Chee WW. A review of contemporary impression materials and techniques. Dent Clin North Am. 2004; 48:445–470.21. Lacy AM, Fukui H, Bellman T, Jendresen MD. Time-dependent accuracy of elastomer impression materials. Part II: Polyether, polysulfides, and polyvinylsiloxane. J Prosthet Dent. 1981; 45:329–333.22. Thongthammachat S, Moore BK, Barco MT 2nd, Hovijitra S, Brown DT, Andres CJ. Dimensional accuracy of dental casts: influence of tray material, impression material, and time. J Prosthodont. 2002; 11:98–108.23. Nassar U, Oko A, Adeeb S, El-Rich M, Flores-Mir C. An in vitro study on the dimensional stability of a vinyl polyether silicone impression material over a prolonged storage period. J Prosthet Dent. 2013; 109:172–178.24. Nassar U, Chow AK. Surface detail reproduction and effect of disinfectant and long-term storage on the dimensional stability of a novel vinyl polyether silicone impression material. J Prosthodont. 2015; 24:494–498.25. American Dental Association Specification no. 19 for Nonaqueous, Elastomeric Dental Impression Materials. J Am Dent Assoc. 1977; 94:733–741.26. Owen CP. An investigation into the compatibility of some irreversible hydrocolloid impression materials and dental gypsum products. Part I. Capacity to record grooves on the international standard die. J Oral Rehabil. 1986; 13:93–103.27. Lepe X, Johnson GH, Berg JC. Surface characteristics of polyether and addition silicone impression materials after long-term disinfection. J Prosthet Dent. 1995; 74:181–186.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An evaluation of the time-dependent dimensional stability of elastomeric impression materials
- A STUDY ON DIMENSIONAL STABILITY OF IMPRESSION MATERIALS FOLLOWING IMMERSION DISINFECTION
- THE EFFECT OF IMMERSION DISINFECTION ON THE DIMENSIONAL STABILITY OF RUBBER IMPRESSION MATERIALS
- A simple and effective method for addition silicone impression disinfection
- Investigation of the effects of storage time on the dimensional accuracy of impression materials using cone beam computed tomography