Cancer Res Treat.
2015 Jan;47(1):78-89. 10.4143/crt.2013.127.
Identification of Hypoxanthine and Phosphoenolpyruvic Acid as Serum Markers of Chemoradiotherapy Response in Locally Advanced Rectal Cancer
- Affiliations
-
- 1Laboratory of Cell Biology, Cancer Research Institute, Seoul National University, Seoul, Korea.
- 2Colorectal Cancer Branch, Research Institute, National Cancer Center, Goyang, Korea. yoo_akh@ncc.re.kr
- 3Department of Radiation Oncology, Soonchunhyang University College of Medicine, Cheonan, Korea.
- KMID: 2380393
- DOI: http://doi.org/10.4143/crt.2013.127
Abstract
- PURPOSE
Patients show variable responses to chemoradiotherapy (CRT), which is generally administered before surgery for locally advanced rectal cancer (LARC). The aim of this study was to identify molecular markers predictive of CRT responses by analysis of low-mass ions (LMIs) in serum of LARC patients.
MATERIALS AND METHODS
LMIs (< 1,000 m/z) in serum obtained before CRT from 73 LARC (cT3-4) patients were profiled using matrix-assisted laser desorption/ionization mass spectrometry. LMIs with higher weighting factors in discriminating CRT responses were selected using principal components analysis and discriminant analysis. Selected LMIs were identified using the Human Metabolome Database. The concentrations of identified LMIs were determined by colorimetric enzyme assay, and compared according to post-CRT pathological stage (ypStage) or Dworak's tumor regression grade (TRG).
RESULTS
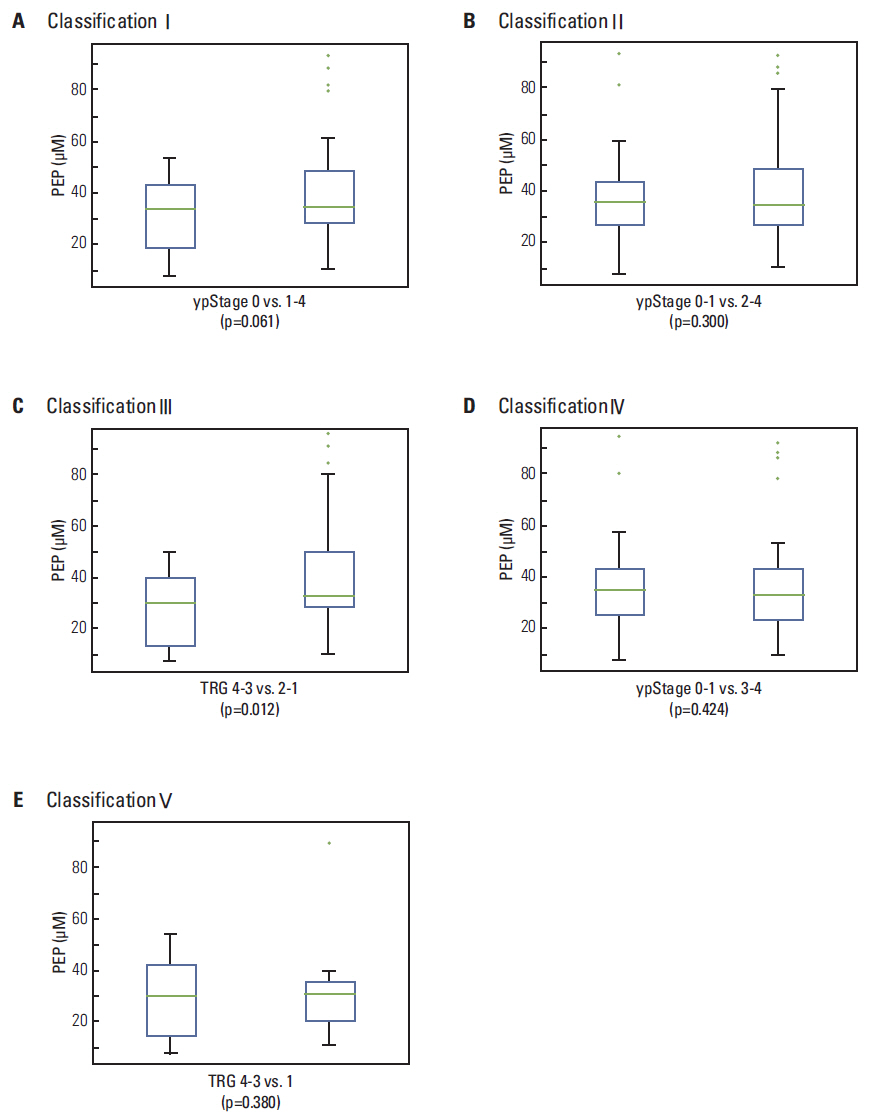
The nine highest-ranking LMIs were selected. Among them, two LMIs with 137.08 and 169.04 m/z were identified as hypoxanthine (HX) and phosphoenolpyruvic acid (PEP), respectively. Higher HX concentration was observed in patients with ypStage 0-1 compared to ypStage 2-4 (p=0.034) or ypStage 3-4 (p=0.030); a similar difference was observed between TRG 4-3 and TRG 1 (p=0.035). HX > 16.0 muM showed significant association with ypStage 0-1 or TRG 4-3 than ypStage 3-4 (p=0.009) or TRG 1 (p=0.024), respectively. In contrast, a significantly lower concentration of PEP was observed in TRG 4-3 compared with TRG 2-1 (p=0.012).
CONCLUSION
Findings of this study demonstrated that serum concentrations of HX and PEP, identified using LMI profiling, may be useful for predicting the CRT response of LARC patients before treatment.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Profiling of Serum Metabolites Using MALDI-TOF and Triple-TOF Mass Spectrometry to Develop a Screen for Ovarian Cancer
Jun Hwa Lee, Yun Hwan Kim, Kyung-Hee Kim, Jae Youl Cho, Sang Myung Woo, Byong Chul Yoo, Seung Cheol Kim
Cancer Res Treat. 2018;50(3):883-893. doi: 10.4143/crt.2017.275.
Reference
-
References
1. Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004; 351:1731–40.
Article2. Yeo SG, Kim DY, Kim TH, Chang HJ, Oh JH, Park W, et al. Pathologic complete response of primary tumor following preoperative chemoradiotherapy for locally advanced rectal cancer: long-term outcomes and prognostic significance of pathologic nodal status (KROG 09-01). Ann Surg. 2010; 252:998–1004.3. Yeo SG, Kim DY. An update on preoperative radiotherapy for locally advanced rectal cancer. J Korean Soc Coloproctol. 2012; 28:179–87.
Article4. Yoo BC, Kong SY, Jang SG, Kim KH, Ahn SA, Park WS, et al. Identification of hypoxanthine as a urine marker for non-Hodgkin lymphoma by low-mass-ion profiling. BMC Cancer. 2010; 10:55.
Article5. Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer;2010.6. Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997; 12:19–23.
Article7. Maas M, Nelemans PJ, Valentini V, Das P, Rodel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010; 11:835–44.
Article8. Kim TH, Chang HJ, Kim DY, Jung KH, Hong YS, Kim SY, et al. Pathologic nodal classification is the most discriminating prognostic factor for disease-free survival in rectal cancer patients treated with preoperative chemoradiotherapy and curative resection. Int J Radiat Oncol Biol Phys. 2010; 77:1158–65.
Article9. Moon SH, Kim DY, Park JW, Oh JH, Chang HJ, Kim SY, et al. Can the new American Joint Committee on Cancer staging system predict survival in rectal cancer patients treated with curative surgery following preoperative chemoradiotherapy? Cancer. 2012; 118:4961–8.
Article10. Choi CH, Kim WD, Lee SJ, Park WY. Clinical predictive factors of pathologic tumor response after preoperative chemoradiotherapy in rectal cancer. Radiat Oncol J. 2012; 30:99–107.
Article11. Yeo SG, Kim DY, Kim TH, Kim SY, Chang HJ, Park JW, et al. Local excision following pre-operative chemoradiotherapyinduced downstaging for selected cT3 distal rectal cancer. Jpn J Clin Oncol. 2010; 40:754–60.
Article12. Vasiliou V, Sandoval M, Backos DS, Jackson BC, Chen Y, Reigan P, et al. ALDH16A1 is a novel non-catalytic enzyme that may be involved in the etiology of gout via proteinprotein interactions with HPRT1. Chem Biol Interact. 2013; 202:22–31.
Article13. Hashimoto H, Kubota M, Shimizu T, Kasai Y, Sano H, Adachi S, et al. Effect of high-dose methotrexate on plasma hypoxanthine and uridine levels in patients with acute leukemia or non-Hodgkin lymphoma in childhood. Leukemia. 1992; 6:1199–202.14. Johnson CH, Patterson AD, Krausz KW, Kalinich JF, Tyburski JB, Kang DW, et al. Radiation metabolomics. 5. Identification of urinary biomarkers of ionizing radiation exposure in nonhuman primates by mass spectrometry-based metabolomics. Radiat Res. 2012; 178:328–40.
Article15. Cooper RA, Kornberg HL. The direct synthesis of phosphoenolpyruvate from pyruvate by Escherichia coli. Proc R Soc Lond B Biol Sci. 1967; 168:263–80.16. Hansen EJ, Juni E. Two routes for synthesis of phosphoenolpyruvate from C4-dicarboxylic acids in Escherichia coli. Biochem Biophys Res Commun. 1974; 59:1204–10.
Article17. Utter MF, Kurahashi K. Purification of oxalacetic carboxylase from chicken liver. J Biol Chem. 1954; 207:787–802.
Article18. Warburg O. On the origin of cancer cells. Science. 1956; 123:309–14.
Article19. Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008; 452:230–3.
Article20. Eigenbrodt E, Reinacher M, Scheefers-Borchel U, Scheefers H, Friis R. Double role for pyruvate kinase type M2 in the expansion of phosphometabolite pools found in tumor cells. Crit Rev Oncog. 1992; 3:91–115.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Update on Preoperative Radiotherapy for Locally Advanced Rectal Cancer
- Surgical issues in locally advanced rectal cancer treated by preoperative chemoradiotherapy
- The Effects and Surgical Morbidity of Preoperative Combined Chemoradiotherapy for Locally Advanced Rectal Cancer
- How Can We Improve the Tumor Response to Preoperative Chemoradiotherapy for Locally Advanced Rectal Cancer?
- Preoperative Concurrent Chemoradiotherapy with Oral Fluoropyrimidine in Locally Advanced Rectal Cancer: How Good Is Good Enough?