J Korean Ophthalmol Soc.
2017 May;58(5):509-515. 10.3341/jkos.2017.58.5.509.
Amoebicidal Effect of Nephrite-containing Contact Lens Storage Case
- Affiliations
-
- 1Department of Ophthalmology, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Korea. Jiel75@hanmail.net
- 2Department of Parasitology, Pusan National University School of Medicine, Yangsan, Korea.
- 3Department of Optometry, Busan Women's College, Busan, Korea.
- 4Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Korea.
- KMID: 2378620
- DOI: http://doi.org/10.3341/jkos.2017.58.5.509
Abstract
- PURPOSE
To compare the amoebicidal effects of nephrite containing contact lens (CL) storage cases with conventional CL storage cases.
METHODS
Acanthamoeba lugdunensis were inoculated onto 5% nephrite containing CL storage cases as well as conventional CL storage cases both with and without silicone hydrogel contact lenses (SHCLs). Then the amount of Acanthamoeba proliferation on CL storage cases and the number of adherent Acanthamoeba on SHCLs were determined and compared. The effects of multipurpose solution (MPS) with and without 1% or 5% nephrite solution on Acanthamoeba adhesion were analyzed.
RESULTS
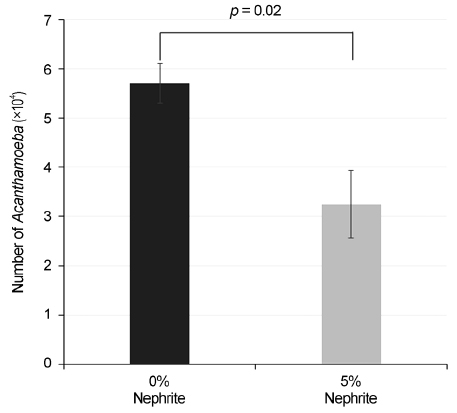
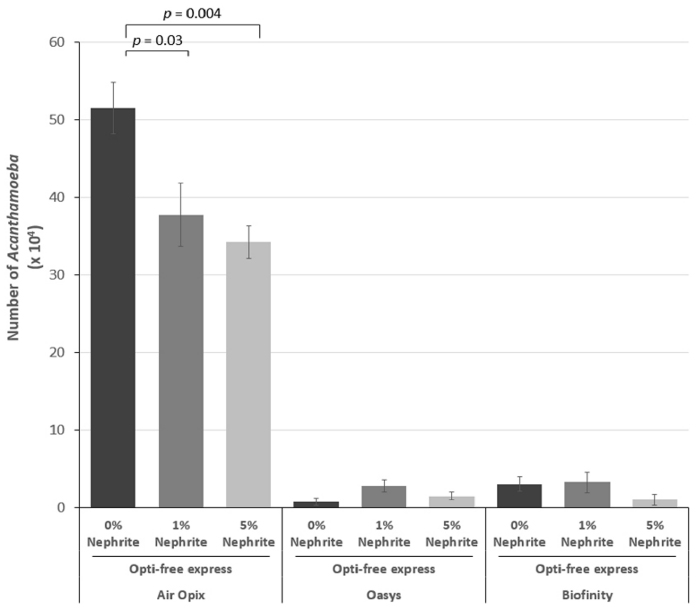
Nephrite containing CL storage cases showed more inhibitory effects on Acanthamoeba proliferation (p = 0.02) and significantly reduced the number of adherent Acanthamoeba on SHCLs compared with conventional CL storage cases, regardless of SHCLs generation (p = 0.001, p = 0.001 and p < 0.001, respectively). The number of adherent Acanthamoeba on the first generation of SHCLs was significantly reduced by MPS with 1% and 5% nephrite solutions (p = 0.03 and p = 0.004, respectively), but the numbers for the second and third generation SHCLs were not.
CONCLUSIONS
Nephrite could be used as a new additive component for CL storage cases and multipurpose solutions to improve the disinfection effects on Acanthamoeba.
Figure
Cited by 1 articles
-
Anti-pseudomonal Effect of a Nephrite-containing Contact Lens Storage Case
Sang Min Lee, Jae Woo Jung, Dong Hyun Lee, Sung Hee Park, Jong Heon Lee, Hak Sun Yu, Yoon Kyung Kim, Ji-Eun Lee
J Korean Ophthalmol Soc. 2018;59(8):724-729. doi: 10.3341/jkos.2018.59.8.724.
Reference
-
1. Naginton J, Watson PG, Playfair TJ, et al. Amoebic infection of the eye. Lancet. 1974; 2:1537–1540.2. Wilhelmus KR. Introduction: the increasing importance of Acanthamoeba. Rev Infect Dis. 1991; 13:Suppl 5. S367–S368.3. Steinemann TL, Fletcher M, Bonny AE, et al. Over-the-counter decorative contact lenses: cosmetic or medical devices? A case series. Eye Contact Lens. 2005; 31:194–200.4. Stehr-Green JK, Bailey TM, Visvesvara GS. The epidemiology of Acanthamoeba keratitis in the United States. Am J Ophthalmol. 1989; 107:331–336.5. Lee SM, Choi YJ, Chung DI. Contamination of Acanthamoeba in contact lens care system. J Korean Ophthalmol Soc. 1997; 38:756–761.6. Moore MB, Ubelaker J, Silvany R, et al. Scanning electron microscopy of Acanthamoeba castellanii: adherence to surface of new and used contact lenses and to human corneal button epithelium. Rev Infect Dis. 1991; 13:Suppl 5. S423.7. Ramachandran L, Janakiraman D, Sharma S, Rao GN. Effect of time and washing on the adhesion of Acanthamoeba to extended wear disposable hydrogel contact lenses. CLAO J. 1997; 23:113–116.8. Kanpolat A, Kalayci D, Arman D, Dürük K. Contamination in contact lens care systems. CLAO J. 1992; 18:105–107.9. Radford CF, Woodward EG, Stapleton F. Contact lens hygiene compliance in a university population. J Br Contact Lens Assoc. 1993; 16:105–111.10. Zhao G, Stevens SE Jr. Multiple parameters for the comprehensive evaluation of the susceptability of Escherichia coli to the silver ion. Biometals. 1998; 11:27–32.11. Amos CF, George MD. Clinical and laboratory testing of a silver-impregnated lens case. Cont Lens Anterior Eye. 2006; 29:247–255.12. Vermeltfoort PB, Hooymans JM, Busscher HJ, van der Mei HC. Bacterial transmission from lens storage cases to contact lenses-Effects of lens care solutons and silver impregnation of cases. J Biomed Mater Res B Appl Biomater. 2008; 87:237–243.13. Scheuer C, Zhao F, Erb T, Orsborn G. Multipurpose solutions and rates of biocidal efficacy. Eye Contact Lens. 2009; 35:88–91.14. Imayasu M, Shimizu H, Shimada S, et al. Effects of multipurpose contact-lens care solutions on adhesion of Pseudomonas aeruginosa to corneal epithelial cells. Eye Contact Lens. 2009; 35:98–104.15. Johnston SP, Sriram R, Qvarnstrom Y, et al. Resistance of Acanthamoeba cysts to disinfection in multiple contact lens solutions. J Clin Microbiol. 2009; 47:2040–2045.16. Kilvington S, Heaselgrave W, Lally JM, et al. Encystment of Acanthamoeba during incubation in multipurpose contact lens disinfectant solutions and experimental formulations. Eye Contact Lens. 2008; 34:133–139.17. Lee JY, Seo BI, Lee IH, Park BJ. A mineralogical geochemical and oriental medical study on nephrite well waters. J Science Education Kyungpook National University. 2001; 25:43–52.18. Yeom MJ, Choi BH, Han DO, et al. In vitro inhibition of pro-inflammatory mediator mRNA expression by nephrite in lipopolysaccharide-induced mouse macrophage cells. Korean J Orient Physiol Pathol. 2004; 18:1622–1627.19. Duguid IG, Dart JK, Morlet N, et al. Outcome of acanthamoeba keratitis treated with polyhexamethyl biguanide and propamidine. Ophthalmology. 1997; 104:1587–1592.20. Stapleton F, Dart JK, Seal DV, Matheson M. Epidemiology of Pseudomonas aeruginosa keratitis in contact lens wearers. Epidemiol Infect. 1995; 114:395–402.21. Giraldez MJ, Serra C, Lira M, et al. Soft contact lens surface profile by atomic force microscopy. Optom Vis Sci. 2010; 87:E475–E481.22. Teichroeb JH, Forrest JA, Ngai V, et al. Imaging protein deposits on contact lens materials. Optom Vis Sci. 2008; 85:1151–1164.23. Beattie TK, Tomlinson A. The effect of surface treatment of silicone hydrogel contact lenses on the attachment of Acanthamoeba castellanii trophozoites. Eye Contact Lens. 2009; 35:316–319.24. Omaña-Molina MA, González-Robles A, Salazar-Villatoro L, et al. Silicone hydrogel contact lenses surface promote Acanthamoeba castellanii trophozoites asherence: qualitative and quantitative analysis. Eye Contact Lens. 2014; 40:132–139.25. Beattie TK, Tomlinson A, McFadyen AK. Attachment of Acanthamoeba to first- and second-generation silicone hydrogel contact lenses. Ophthalmology. 2006; 113:117–125.26. Shayani Rad M, Khameneh B, Sabeti , et al. Antibacterial activity of silver nanoparticle-loaded soft contact lens materials: the effect of monomer composition. Curr Eye Res. 2016; 41:1286–1293.27. Dantam J, Zhu H, Stapleton F. Biocidal efficacy of silver-impregnated contact lens storage cases in vitro. Invest Ophthalmol Vis Sci. 2011; 52:51–57.28. Kilvington S. Moist-heat disinfection of pathogenic Acanthamoeba cysts. Lett Appl Microbiol. 1989; 9:187–189.29. Lindquist TD, Doughman DJ, Rubenstein JB, et al. Acanthamoeba-contaminated hydrogel contact lenses. Susceptibility to disinfection. Cornea. 1988; 7:300–303.30. Hughes R, Kilvington S. Comparison of hydrogen peroxide contact lens disinfection systems and solutions against Acanthamoeba polyphaga. Antimicrob Agents Chemother. 2001; 45:2038–2043.31. Dutot M, Reveneau E, Pauloin T, et al. Multipurpose solutions and contact lens: modulation of cytotoxicity and apoptosis on the ocular surface. Cornea. 2010; 29:541–549.32. Lee GH, Yu HS, Lee JE. Effects of multipurpose solutions on the adhesion of Acanthamoeba to rigid gas permeable contact lenses. Ophthalmic Physiol Opt. 2016; 36:93–99.33. Moon EK, Park HR, Quan FS, Kong HH. Efficacy of Korean multipurpose contact lens disinfecting solutions against Acanthamoeba castellanii. Korean J Parasitol. 2016; 54:697.34. Lee GH, Lee JE, Park MK, Yu HS. Adhesion of Acanthamoeba on silicone hydrogel contact lenses. Cornea. 2016; 35:663–668.35. Han DO, Choi BH, Lee HJ, et al. In vivo studies on anti-inflammatory activity of nephrite. Korean J Orient Physiol Pathol. 2005; 19:977–981.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-pseudomonal Effect of a Nephrite-containing Contact Lens Storage Case
- Anti-staphylococcal Effect of a Nephrite-containing Contact Lens Storage Case
- Contamination of Contact Lens or Contact Lens Storage Case in Contact Lens Related Infectious Keratitis
- Microbial Contamination of Contact Lens Storage Cases in Contact Lens-induced Keratitis Patients
- Effect of 2, 6-Dichlorobenzonitrile on Amoebicidal Activity of Multipurpose Contact Lens Disinfecting Solutions