Brain Tumor Res Treat.
2017 Apr;5(1):1-9. 10.14791/btrt.2017.5.1.1.
Bevacizumab in Recurrent Glioma: Patterns of Treatment Failure and Implications
- Affiliations
-
- 1Department of Radiology and Biomedical Imaging, University of California, San Francisco, CA, USA. yi.li@ucsf.edu
- 2Department of Radiology, University of Chicago, Chicago, IL, USA.
- 3Department of Neurology, University of California, San Francisco, CA, USA.
- KMID: 2378298
- DOI: http://doi.org/10.14791/btrt.2017.5.1.1
Abstract
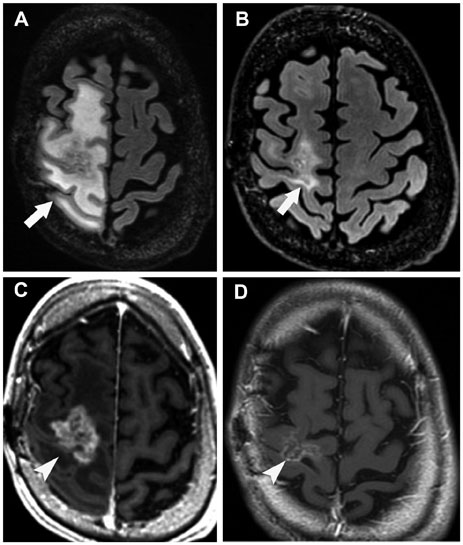
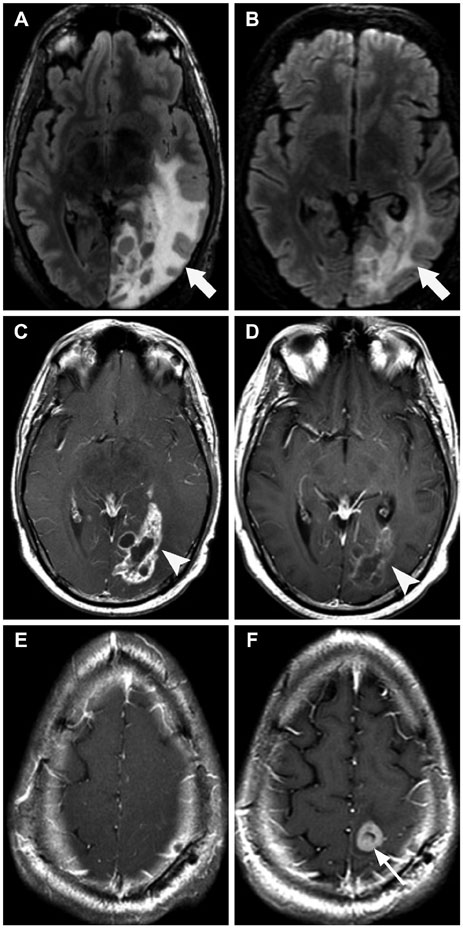
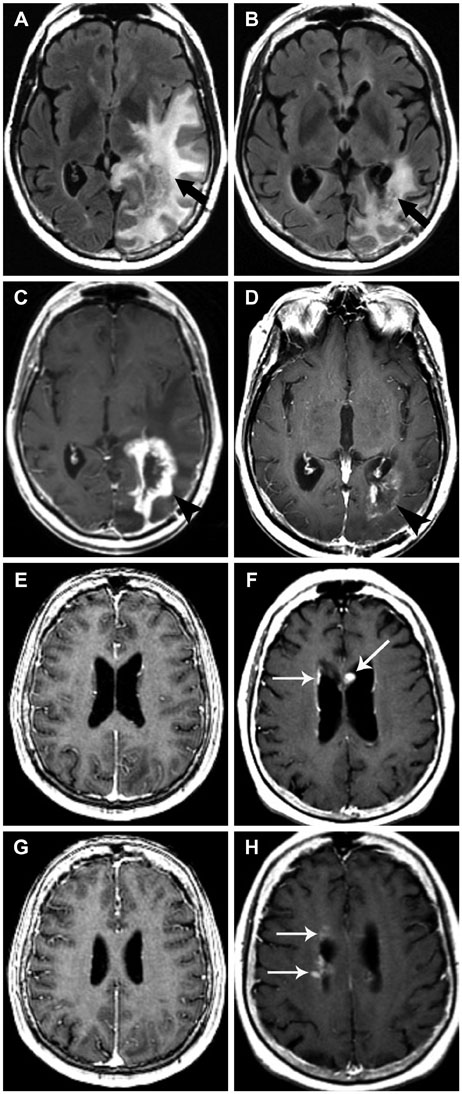
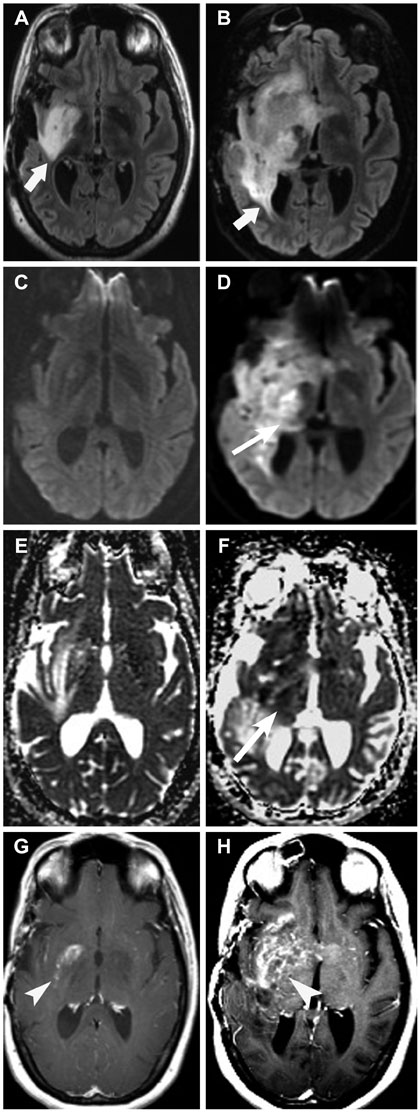
- Glioblastoma, the most common primary malignant brain tumor in adults, is highly aggressive and associated with a poor prognosis. Bevacizumab, a monoclonal antibody against the vascular endothelial growth factor receptor, has increasingly been used in the treatment of recurrent glioblastoma. It has achieved excellent rates of radiographic response, but most patients will progress after only a few months. Upon recurrence, tumors may not enhance, secondary to vascular normalization. We describe four patterns of radiographic progression commonly associated with Bevacizumab failure: 1) Distant enhancing tumor, 2) Local tumor progression without enhancement, 3) Diffuse gliomatosis-like infiltration, and 4) Local or multifocal progression, with enhancement. Some have noted an increased incidence of distant or diffuse disease upon recurrence, suggestive of a transition to a more aggressive phenotype, but a review of the literature suggests there is no conclusive evidence that Bevacizumab treatment is associated with an increased rate of distant or diffuse recurrence.
Keyword
MeSH Terms
Figure
Reference
-
1. Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006; 2:494–503.
Article2. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–996.
Article3. Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013; 31:4085–4091.
Article4. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2014; 16:Suppl 4. iv1–iv63.
Article5. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009; 27:4733–4740.
Article6. Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009; 27:740–745.
Article7. Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008; 70:779–787.
Article8. Pope WB, Xia Q, Paton VE, et al. Patterns of progression in patients with recurrent glioblastoma treated with bevacizumab. Neurology. 2011; 76:432–437.
Article9. Kargiotis O, Rao JS, Kyritsis AP. Mechanisms of angiogenesis in gliomas. J Neurooncol. 2006; 78:281–293.
Article10. Rubenstein JL, Kim J, Ozawa T, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000; 2:306–314.
Article11. de Groot JF, Fuller G, Kumar AJ, et al. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010; 12:233–242.
Article12. Lu KV, Chang JP, Parachoniak CA, et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell. 2012; 22:21–35.
Article13. Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008; 8:579–591.
Article14. Thompson EM, Frenkel EP, Neuwelt EA. The paradoxical effect of bevacizumab in the therapy of malignant gliomas. Neurology. 2011; 76:87–93.
Article15. Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006; 66:1258–1260.
Article16. Ali SA, McHayleh WM, Ahmad A, et al. Bevacizumab and irinotecan therapy in glioblastoma multiforme: a series of 13 cases. J Neurosurg. 2008; 109:268–272.
Article17. Bokstein F, Shpigel S, Blumenthal DT. Treatment with bevacizumab and irinotecan for recurrent high-grade glial tumors. Cancer. 2008; 112:2267–2273.
Article18. Kang TY, Jin T, Elinzano H, Peereboom D. Irinotecan and bevacizumab in progressive primary brain tumors, an evaluation of efficacy and safety. J Neurooncol. 2008; 89:113–118.
Article19. Narayana A, Kelly P, Golfinos J, et al. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survival. J Neurosurg. 2009; 110:173–180.
Article20. Thompson EM, Dosa E, Kraemer DF, Neuwelt EA. Treatment with bevacizumab plus carboplatin for recurrent malignant glioma. Neurosurgery. 2010; 67:87–93.
Article21. Vredenburgh JJ, Desjardins A, Herndon JE 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007; 13:1253–1259.
Article22. Vredenburgh JJ, Desjardins A, Herndon JE 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007; 25:4722–4729.
Article23. Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014; 15:943–953.
Article24. Brandes AA, Finocchiaro G, Zagonel V, et al. AT-11. Final results from the randomized phase II trial avareg (ML25739) with bevacizumab (BEV) or fotemustine (FTM) in recurrent GBM. Neuro Oncol. 2014; 16:suppl 5. v10.25. Wick W, Brandes AA, Gorlia T, et al. EORTC 26101 phase III trial exploring the combination of bevacizumab and lomustine in patients with first progression of a glioblastoma. J Clin Oncol. 2016; 34:suppl. abstract 2001.
Article26. Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008; 8:592–603.
Article27. Kunkel P, Ulbricht U, Bohlen P, et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001; 61:6624–6628.28. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990; 8:1277–1280.
Article29. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010; 28:1963–1972.
Article30. Claes A, Gambarota G, Hamans B, et al. Magnetic resonance imaging-based detection of glial brain tumors in mice after antiangiogenic treatment. Int J Cancer. 2008; 122:1981–1986.
Article31. Shapiro LQ, Beal K, Goenka A, et al. Patterns of failure after concurrent bevacizumab and hypofractionated stereotactic radiation therapy for recurrent high-grade glioma. Int J Radiat Oncol Biol Phys. 2013; 85:636–642.
Article32. Prados M, Cloughesy T, Samant M, et al. Response as a predictor of survival in patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011; 13:143–151.
Article33. Nowosielski M, Wiestler B, Goebel G, et al. Progression types after antiangiogenic therapy are related to outcome in recurrent glioblastoma. Neurology. 2014; 82:1684–1692.
Article34. Zuniga RM, Torcuator R, Jain R, et al. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol. 2009; 91:329–336.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radiological Recurrence Patterns after Bevacizumab Treatment of Recurrent High-Grade Glioma: A Systematic Review and Meta-Analysis
- Post-bevacizumab Clinical Outcomes and the Impact of Early Discontinuation of Bevacizumab in Patients with Recurrent Malignant Glioma
- Recurrent Conjunctival Granuloma Treated with Subconjunctival Bevacizumab
- Clinical Results After Application of Bevacizumab in Recurrent Pterygium
- Surgical Experience of Multiple Recurrent Astrocytoma: Case Report