Cancer Res Treat.
2017 Apr;49(2):416-422. 10.4143/crt.2016.121.
Phase II Study of Irinotecan and Cisplatin Combination Chemotherapy in Metastatic, Unresectable Esophageal Cancer
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea. bhumsuk@snu.ac.kr
- 2Department of Internal Medicine, Institute of Health Sciences, Gyeongsang National University Hospital, Gyeongsang National University School of Medicine, Jinju, Korea.
- 3Department of Internal Medicine, SMG-SNU Boramae Medical Center, Seoul, Korea.
- 4Department of Hematology-Oncology, Inje University Haeundae Paik Hospital, Busan, Korea.
- 5Department of Internal Medicine, Kosin University Gospel Hospital, Busan, Korea.
- 6Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea.
- 7Department of Hematology-Oncology, Keimyung University School of Medicine, Daegu, Korea.
- 8Department of Internal Medicine, Chungnam National University College of Medicine, Daejeon, Korea.
- 9Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 2378113
- DOI: http://doi.org/10.4143/crt.2016.121
Abstract
- PURPOSE
The objective of this multicenter phase II study was to evaluate the efficacy and safety of irinotecan and cisplatin combination chemotherapy in metastatic, unresectable esophageal cancer.
MATERIALS AND METHODS
Patients were treated with irinotecan 65 mg/m² and cisplatin 30 mg/m² on days 1 and 8 of each 21-day treatment cycle. The primary endpoint was response rate, and secondary endpoints were survival, duration of response, initial metabolic response rate, and toxicity.
RESULTS
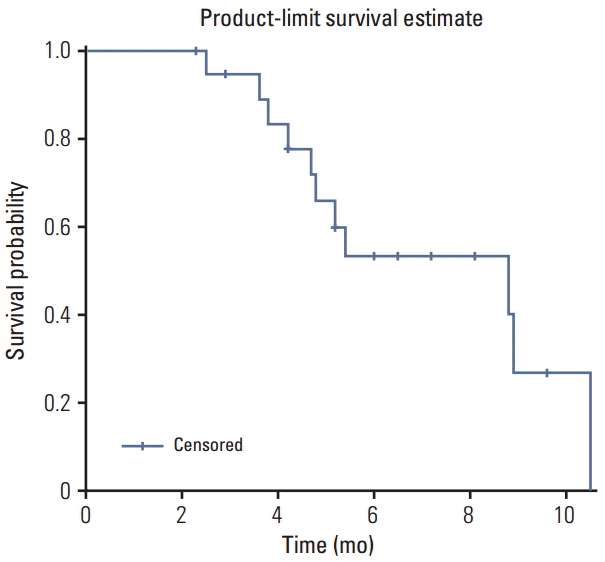
A total of 27 patients with squamous cell histology were enrolled in the study. The median age of the patients was 61 years. The objective response rate of the 20 patients in the perprotocol group was 30.0% (90% confidence interval [CI], 13.2 to 46.9). The median follow-up duration was 10.0 months, and the median progression-free survival and overall survival were 4.5 months (95% CI, 1.6 to 6.2) and 8.8 months (95% CI, 4.7 to 10.5), respectively. Four of 13 patients (30.8%) evaluated showed initial metabolic response. The median duration of response for partial responders was 5.0 months (range, 3.4 to 8.0 months). The following grade 3/4 treatment-related hematologic toxicities were reported: neutropenia (40.7%), anaemia (22.2%), and thrombocytopenia (7.4%). Two patients experienced febrile neutropenia. The most common grade 3/4 non-hematologic toxicities were asthenia (14.8%) and diarrhoea (11.1%).
CONCLUSION
Irinotecan and cisplatin combination chemotherapy showed modest anti-tumour activity and manageable toxicity for patients with metastatic, unresectable esophageal cancer.
MeSH Terms
Figure
Reference
-
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.
Article2. Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015; 47:127–41.
Article3. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013; 381:400–12.
Article4. Bleiberg H, Conroy T, Paillot B, Lacave AJ, Blijham G, Jacob JH, et al. Randomised phase II study of cisplatin and 5-fluorouracil (5-FU) versus cisplatin alone in advanced squamous cell oesophageal cancer. Eur J Cancer. 1997; 33:1216–20.
Article5. Muhr-Wilkenshoff F, Hinkelbein W, Ohnesorge I, Wolf KJ, Riecken EO, Zeitz M, et al. A pilot study of irinotecan (CPT-11) as single-agent therapy in patients with locally advanced or metastatic esophageal carcinoma. Int J Colorectal Dis. 2003; 18:330–4.
Article6. Enzinger PC, Kulke MH, Clark JW, Ryan DP, Kim H, Earle CC, et al. A phase II trial of irinotecan in patients with previously untreated advanced esophageal and gastric adenocarcinoma. Dig Dis Sci. 2005; 50:2218–23.
Article7. Wolff K, Wein A, Reulbach U, Mannlein G, Bruckl V, Meier C, et al. Weekly high-dose 5-fluorouracil as a 24-h infusion and sodium folinic acid (AIO regimen) plus irinotecan in patients with locally advanced nonresectable and metastatic adenocarcinoma or squamous cell carcinoma of the oesophagus: a phase II trial. Anticancer Drugs. 2009; 20:165–73.
Article8. Enzinger PC, Ryan DP, Clark JW, Muzikansky A, Earle CC, Kulke MH, et al. Weekly docetaxel, cisplatin, and irinotecan (TPC): results of a multicenter phase II trial in patients with metastatic esophagogastric cancer. Ann Oncol. 2009; 20:475–80.
Article9. Lee DH, Kim HT, Han JY, Lee SY, Yoon SJ, Kim HY, et al. A phase II trial of modified weekly irinotecan and cisplatin for chemotherapy-naive patients with metastatic or recurrent squamous cell carcinoma of the esophagus. Cancer Chemother Pharmacol. 2008; 61:83–8.10. Ilson DH, Saltz L, Enzinger P, Huang Y, Kornblith A, Gollub M, et al. Phase II trial of weekly irinotecan plus cisplatin in advanced esophageal cancer. J Clin Oncol. 1999; 17:3270–5.
Article11. Assersohn L, Brown G, Cunningham D, Ward C, Oates J, Waters JS, et al. Phase II study of irinotecan and 5-fluorouracil/leucovorin in patients with primary refractory or relapsed advanced oesophageal and gastric carcinoma. Ann Oncol. 2004; 15:64–9.
Article12. Burtness B, Gibson M, Egleston B, Mehra R, Thomas L, Sipples R, et al. Phase II trial of docetaxel-irinotecan combination in advanced esophageal cancer. Ann Oncol. 2009; 20:1242–8.
Article13. Ilson DH. Phase II trial of weekly irinotecan/cisplatin in advanced esophageal cancer. Oncology (Williston Park). 2004; 18(14 Suppl 14):22–5.14. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article15. Elliott JA, O'Farrell NJ, King S, Halpenny D, Malik V, Muldoon C, et al. Value of CT-PET after neoadjuvant chemoradiation in the prediction of histological tumour regression, nodal status and survival in oesophageal adenocarcinoma. Br J Surg. 2014; 101:1702–11.
Article16. Tamandl D, Gore RM, Fueger B, Kinsperger P, Hejna M, Paireder M, et al. Change in volume parameters induced by neoadjuvant chemotherapy provide accurate prediction of overall survival after resection in patients with oesophageal cancer. Eur Radiol. 2016; 26:311–21.
Article17. Pan L, Gu P, Huang G, Xue H, Wu S. Prognostic significance of SUV on PET/CT in patients with esophageal cancer: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2009; 21:1008–15.
Article18. Gillies RS, Middleton MR, Blesing C, Patel K, Warner N, Marshall RE, et al. Metabolic response at repeat PET/CT predicts pathological response to neoadjuvant chemotherapy in oesophageal cancer. Eur Radiol. 2012; 22:2035–43.
Article19. Kukar M, Alnaji RM, Jabi F, Platz TA, Attwood K, Nava H, et al. Role of repeat 18F-fluorodeoxyglucose positron emission tomography examination in predicting pathologic response following neoadjuvant chemoradiotherapy for esophageal adenocarcinoma. JAMA Surg. 2015; 150:555–62.20. Shimoyama S. Pharmacogenetics of irinotecan: An ethnicity-based prediction of irinotecan adverse events. World J Gastrointest Surg. 2010; 2:14–21.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phase II Study of Irinotecan Plus Cisplatin as First Line therapy in Extensive Small-Cell Lung Cancer
- Novel Palliative Chemotherapy for Cholangiocarcinoma
- Phase II trial of 5-FU, etoposide, cisplatin (FEP) combination chemotherapy in unresectable non-small cell lung cancer
- Phase II Study of Irinotecan, High-dose 5-fluorouracil, and Leucovorin Combination Chemotherapy in Taxane and Cisplatin-based Chemotherapy-refractory Metastatic Gastric Cancer
- Chemotherapy of Advanced Gastric Cancer