Korean J Physiol Pharmacol.
2017 May;21(3):279-286. 10.4196/kjpp.2017.21.3.279.
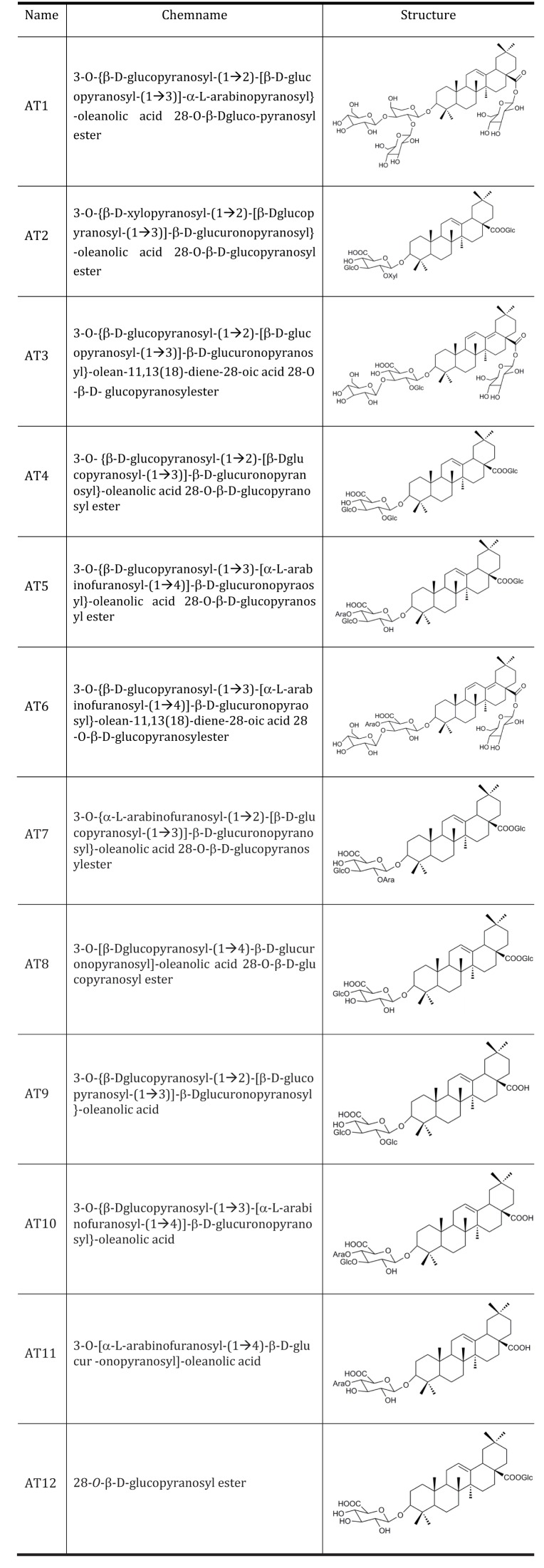
Identification of AMPK activator from twelve pure compounds isolated from Aralia Taibaiensis: implication in antihyperglycemic and hypolipidemic activities
- Affiliations
-
- 1Department of Pharmacy, Xijing Hospital, Fourth Military Medical University, Xi' an 710032, P. R. China. aidongwen71@hotmail.com miaomiaoxi02@hotmail.com
- 2Department of Pharmacology, Chungnam National University, Daejon 34134, Korea.
- KMID: 2376955
- DOI: http://doi.org/10.4196/kjpp.2017.21.3.279
Abstract
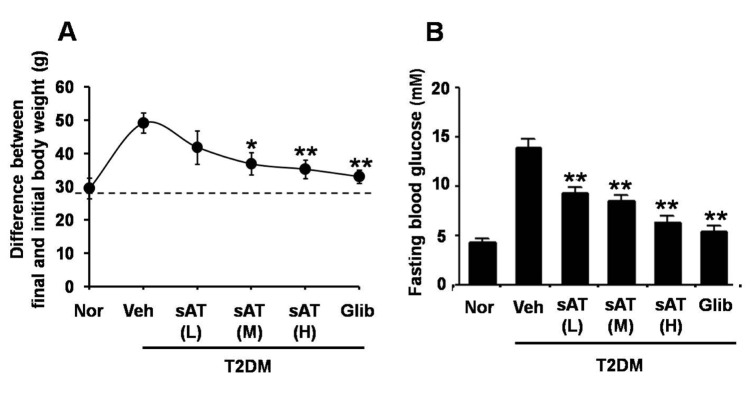
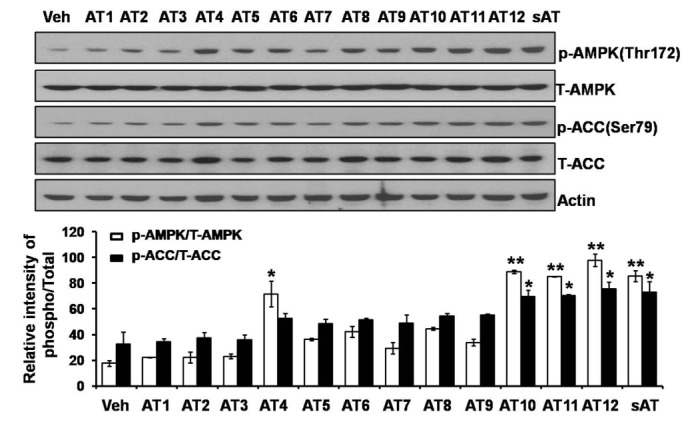
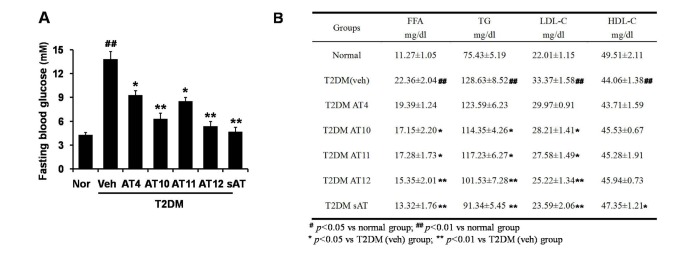
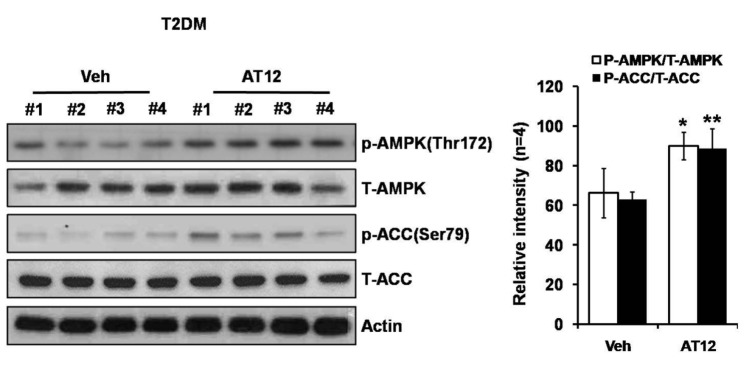
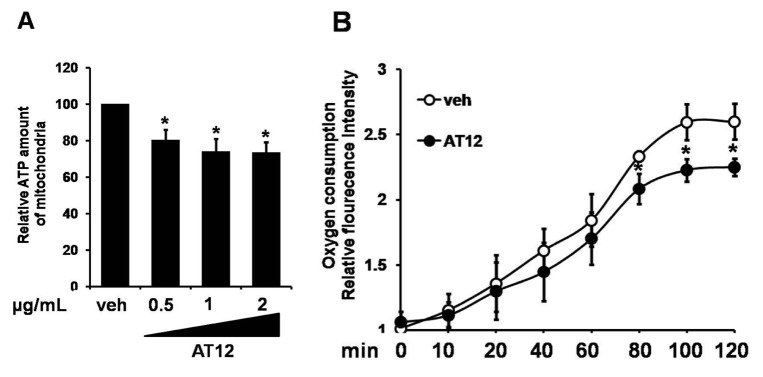
- The root bark extract of Aralia taibaiensis is used traditionally for the treatment of diabetes mellitus in China. The total saponin extracted from Aralia Taibaiensis (sAT) has effective combined antihyperglycemic and hypolipidemic activities in experimental type 2 diabetic rats. However, the active compounds have not yet been fully investigated. In the present study, we examined effects of twelve triterpenoid saponins on AMP-activated protein kinase (AMPK) activation, and found that compound 28-O-β-D-glucopyranosyl ester (AT12) significantly increased phosphorylation of AMPK and Acetyl-CoA carboxylase (ACC). AT12 effectively decreased blood glucose, triglyceride (TG), free fatty acid (FFA) and low density lipoprotein-cholesterol (LDL-C) levels in the rat model of type 2 diabetes mellitus (T2DM). The mechanism by which AT12 activated AMPK was subsequently investigated. Intracellular ATP level and oxygen consumption were significantly reduced by AT12 treatment. The findings suggested AT12 was a novel AMPK activator, and could be useful for the treatment of metabolic diseases.
Keyword
MeSH Terms
-
Acetyl-CoA Carboxylase
Adenosine Triphosphate
AMP-Activated Protein Kinases*
Animals
Aralia*
Blood Glucose
China
Diabetes Mellitus
Diabetes Mellitus, Type 2
Metabolic Diseases
Models, Animal
Oxygen Consumption
Phosphorylation
Rats
Saponins
Triglycerides
AMP-Activated Protein Kinases
Acetyl-CoA Carboxylase
Adenosine Triphosphate
Blood Glucose
Saponins
Figure
Reference
-
1. Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009; 9:407–416. PMID: 19416711.
Article2. Adachi Y, Kanbayashi Y, Harata I, Ubagai R, Takimoto T, Suzuki K, Miwa T, Noguchi Y. Petasin activates AMP-activated protein kinase and modulates glucose metabolism. J Nat Prod. 2014; 77:1262–1269. PMID: 24871354.
Article3. Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006; 444:337–342. PMID: 17086191.
Article4. Xi M, Hai C, Tang H, Wen A, Chen H, Liu R, Liang X, Chen M. Antioxidant and antiglycation properties of triterpenoid saponins from Aralia taibaiensis traditionally used for treating diabetes mellitus. Redox Rep. 2010; 15:20–28. PMID: 20196925.5. Weng Y, Yu L, Cui J, Zhu YR, Guo C, Wei G, Duan JL, Yin Y, Guan Y, Wang YH, Yang ZF, Xi MM, Wen AD. Antihyperglycemic, hypolipidemic and antioxidant activities of total saponins extracted from Aralia taibaiensis in experimental type 2 diabetic rats. J Ethnopharmacol. 2014; 152:553–560. PMID: 24524879.
Article6. Abdel-Zaher AO, Salim SY, Assaf MH, Abdel-Hady RH. Antidiabetic activity and toxicity of Zizyphus spina-christi leaves. J Ethnopharmacol. 2005; 101:129–138. PMID: 16009520.
Article7. Lee KT, Sohn IC, Kim DH, Choi JW, Kwon SH, Park HJ. Hypoglycemic and hypolipidemic effects of tectorigenin and kaikasaponin III in the streptozotocin-lnduced diabetic rat and their antioxidant activity in vitro. Arch Pharm Res. 2000; 23:461–466. PMID: 11059824.8. Oishi Y, Sakamoto T, Udagawa H, Taniguchi H, Kobayashi-Hattori K, Ozawa Y, Takita T. Inhibition of increases in blood glucose and serum neutral fat by Momordica charantia saponin fraction. Biosci Biotechnol Biochem. 2007; 71:735–740. PMID: 17341830.9. Xi M, Hai C, Tang H, Chen M, Fang K, Liang X. Antioxidant and antiglycation properties of total saponins extracted from traditional Chinese medicine used to treat diabetes mellitus. Phytother Res. 2008; 22:228–237. PMID: 17886226.
Article10. Tang HF, Yi YH, Wang ZZ, Hu WJ, Li YQ. Studies on the triterpenoid saponins of the root bark of Aralia taibaiensis. Yao Xue Xue Bao. 1996; 31:517–523. PMID: 9772693.11. Tang HF, Yi YH, Wang ZZ, Jiang YP, Li YQ. Oleanolic acid saponins from the root bark of Aralia taibaiensis. Yao Xue Xue Bao. 1997; 32:685–690. PMID: 11596294.12. Buhl ES, Jessen N, Pold R, Ledet T, Flyvbjerg A, Pedersen SB, Pedersen O, Schmitz O, Lund S. Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes. 2002; 51:2199–2206. PMID: 12086950.
Article13. Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest. 2013; 123:2764–2772. PMID: 23863634.
Article14. Park SY, Kim MH, Ahn JH, Lee SJ, Lee JH, Eum WS, Choi SY, Kwon HY. The Stimulatory effect of essential fatty acids on glucose uptake involves both Akt and AMPK activation in C2C12 skeletal muscle cells. Korean J Physiol Pharmacol. 2014; 18:255–261. PMID: 24976766.
Article15. Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5' AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998; 47:1369–1373. PMID: 9703344.
Article16. Sullivan JE, Brocklehurst KJ, Marley AE, Carey F, Carling D, Beri RK. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 1994; 353:33–36. PMID: 7926017.
Article17. Fujii N, Hirshman MF, Kane EM, Ho RC, Peter LE, Seifert MM, Goodyear LJ. AMP-activated protein kinase alpha2 activity is not essential for contraction- and hyperosmolarity-induced glucose transport in skeletal muscle. J Biol Chem. 2005; 280:39033–39041. PMID: 16186119.18. Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008; 134:405–415. PMID: 18674809.19. Kim JE, Choi HC. Losartan inhibits vascular smooth muscle cell proliferation through activation of amp-activated protein kinase. Korean J Physiol Pharmacol. 2010; 14:299–304. PMID: 21165328.
Article20. Carling D, Mayer FV, Sanders MJ, Gamblin SJ. AMP-activated protein kinase: nature's energy sensor. Nat Chem Biol. 2011; 7:512–518. PMID: 21769098.
Article21. Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998; 67:821–855. PMID: 9759505.
Article22. Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, Roden M, Gnaiger E, Nohl H, Waldhäusl W, Fürnsinn C. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes. 2004; 53:1052–1059. PMID: 15047621.23. Sebbagh M, Olschwang S, Santoni MJ, Borg JP. The LKB1 complex-AMPK pathway: the tree that hides the forest. Fam Cancer. 2011; 10:415–424. PMID: 21656073.
Article24. Carling D, Sanders MJ, Woods A. The regulation of AMP-activated protein kinase by upstream kinases. Int J Obes (Lond). 2008; 32(Suppl 4):S55–S59.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- AMPK activators: mechanisms of action and physiological activities
- Effects of Compounds from Physalis angulata on Fatty Acid Synthesis and Glucose Metabolism in HepG2 Cells via the AMP-activated Protein Kinase Pathway
- Humanin suppresses receptor activator of nuclear factor-κB ligand-induced osteoclast differentiation via AMP-activated protein kinase activation
- AMPK and Exercise: Glucose Uptake and Insulin Sensitivity
- Effects of AMP-activated Protein Kinase Activating Compounds and Its Mechanism