Investig Magn Reson Imaging.
2017 Mar;21(1):9-19. 10.13104/imri.2017.21.1.9.
Differentiation between Glioblastoma and Primary Central Nervous System Lymphoma Using Dynamic Susceptibility Contrast-Enhanced Perfusion MR Imaging: Comparison Study of the Manual versus Semiautomatic Segmentation Method
- Affiliations
-
- 1College of Medicine, Seoul National University, Seoul, Korea.
- 2Department of Radiology, Seoul National University College of Medicine, Seoul, Korea. verocay@snuh.org
- 3Center for Nanoparticle Research, Institute for Basic Science (IBS), Seoul National University, Seoul, Korea.
- 4School of Chemical and Biological Engineering, Seoul National University, Seoul, Korea.
- 5Department of Neurology, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Internal Medicine, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 7Department of Neurosurgery, Biomedical Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 8Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- 9Department of Radiation Oncology, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2376264
- DOI: http://doi.org/10.13104/imri.2017.21.1.9
Abstract
- BACKGROUND
Normalized cerebral blood volume (nCBV) can be measured using manual or semiautomatic segmentation method. However, the difference in diagnostic performance on brain tumor differentiation between differently measured nCBV has not been evaluated. PURPOSE: To compare the diagnostic performance of manually obtained nCBV to that of semiautomatically obtained nCBV on glioblastoma (GBM) and primary central nervous system lymphoma (PCNSL) differentiation.
MATERIALS AND METHODS
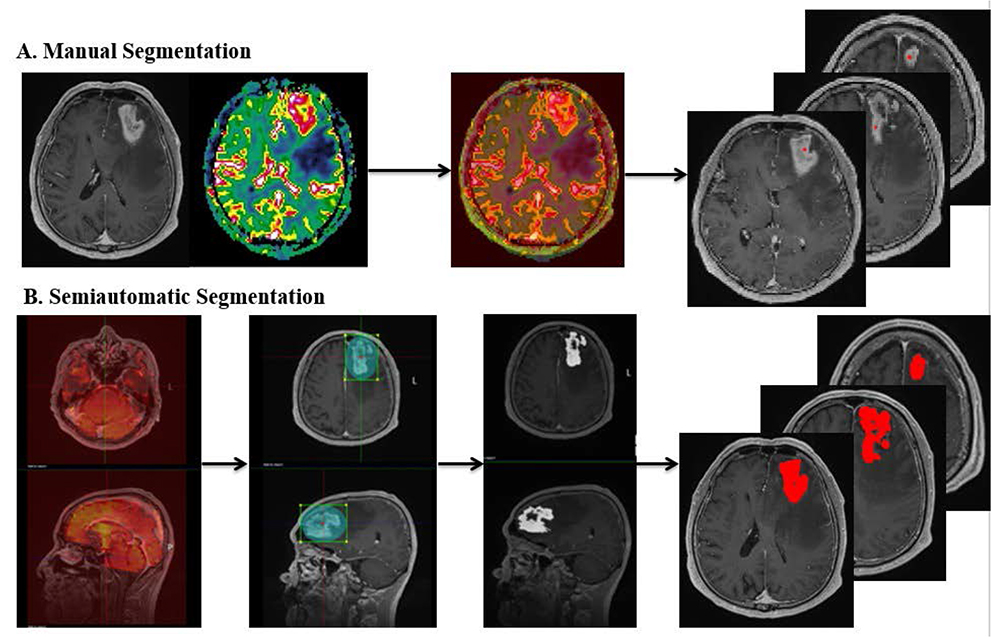
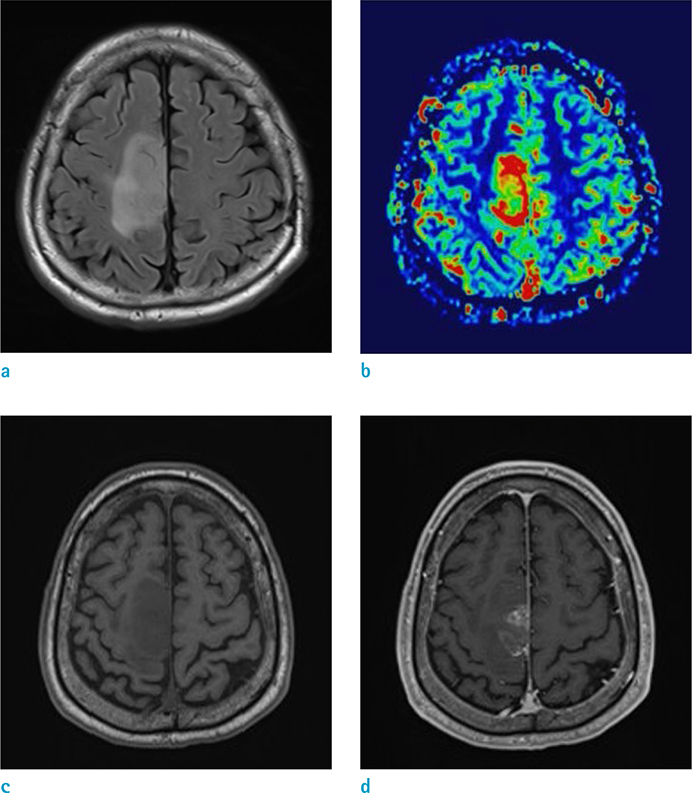
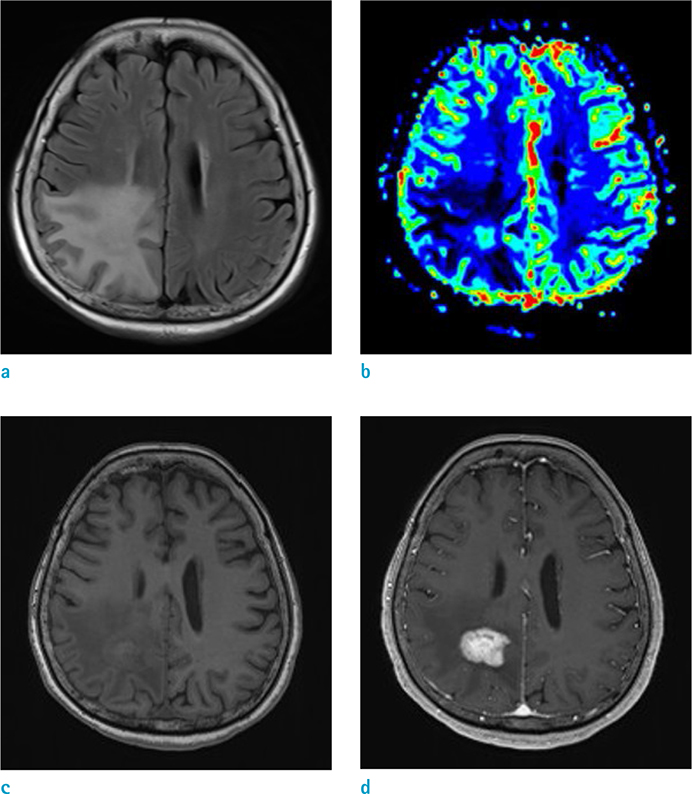
Histopathologically confirmed forty GBM and eleven PCNSL patients underwent 3T MR imaging with dynamic susceptibility contrast-enhanced perfusion MR imaging before any treatment or biopsy. Based on the contrast-enhanced T1-weighted imaging, the mean nCBV (mCBV) was measured using the manual method (manual mCBV), random regions of interest (ROIs) placement by the observer, or the semiautomatic segmentation method (semiautomatic mCBV). The volume of enhancing portion of the tumor was also measured during semiautomatic segmentation process. T-test, ROC curve analysis, Fisher's exact test and multivariate regression analysis were performed to compare the value and evaluate the diagnostic performance of each parameter.
RESULTS
GBM showed a higher enhancing volume (P = 0.0307), a higher manual mCBV (P = 0.018) and a higher semiautomatic mCBV (P = 0.0111) than that of the PCNSL. Semiautomatic mCBV had the highest value (0.815) for the area under the curve (AUC), however, the AUCs of the three parameters were not significantly different from each other. The semiautomatic mCBV was the best independent predictor for the GBM and PCNSL differential diagnosis according to the stepwise multiple regression analysis.
CONCLUSION
We found that the semiautomatic mCBV could be a better predictor than the manual mCBV for the GBM and PCNSL differentiation. We believe that the semiautomatic segmentation method can contribute to the advancement of perfusion based brain tumor evaluation.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Dynamic Susceptibility Contrast (DSC) Perfusion MR in the Prediction of Long-Term Survival of Glioblastomas (GBM): Correlation with MGMT Promoter Methylation and 1p/19q Deletions
Yong Wonn Kwon, Won-Jin Moon, Mina Park, Hong Gee Roh, Young Cho Koh, Sang Woo Song, Jin Woo Choi
Investig Magn Reson Imaging. 2018;22(3):158-167. doi: 10.13104/imri.2018.22.3.158.
Reference
-
1. Olson JE, Janney CA, Rao RD, et al. The continuing increase in the incidence of primary central nervous system non-Hodgkin lymphoma: a surveillance, epidemiology, and end results analysis. Cancer. 2002; 95:1504–1510.2. Fisher JL, Schwartzbaum JA, Wrensch M, Wiemels JL. Epidemiology of brain tumors. Neurol Clin. 2007; 25:867–890.3. DeAngelis LM. Brain tumors. N Engl J Med. 2001; 344:114–123.4. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007; 114:97–109.5. Faehndrich J, Weidauer S, Pilatus U, Oszvald A, Zanella FE, Hattingen E. Neuroradiological viewpoint on the diagnostics of space-occupying brain lesions. Clin Neuroradiol. 2011; 21:123–139.6. Provenzale JM, Mukundan S, Barboriak DP. Diffusion-weighted and perfusion MR imaging for brain tumor characterization and assessment of treatment response. Radiology. 2006; 239:632–649.7. Kickingereder P, Wiestler B, Sahm F, et al. Primary central nervous system lymphoma and atypical glioblastoma: multiparametric differentiation by using diffusion-, perfusion-, and susceptibility-weighted MR imaging. Radiology. 2014; 272:843–850.8. Calli C, Kitis O, Yunten N, Yurtseven T, Islekel S, Akalin T. Perfusion and diffusion MR imaging in enhancing malignant cerebral tumors. Eur J Radiol. 2006; 58:394–403.9. Hartmann M, Heiland S, Harting I, et al. Distinguishing of primary cerebral lymphoma from high-grade glioma with perfusion-weighted magnetic resonance imaging. Neurosci Lett. 2003; 338:119–122.10. Rollin N, Guyotat J, Streichenberger N, Honnorat J, Tran Minh VA, Cotton F. Clinical relevance of diffusion and perfusion magnetic resonance imaging in assessing intra-axial brain tumors. Neuroradiology. 2006; 48:150–159.11. Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol. 2011; 32:984–992.12. Cha S, Knopp EA, Johnson G, Wetzel SG, Litt AW, Zagzag D. Intracranial mass lesions: dynamic contrast-enhanced susceptibility-weighted echo-planar perfusion MR imaging. Radiology. 2002; 223:11–29.13. Suh CH, Kim HS, Lee SS, et al. Atypical imaging features of primary central nervous system lymphoma that mimics glioblastoma: utility of intravoxel incoherent motion MR imaging. Radiology. 2014; 272:504–513.14. Blasel S, Jurcoane A, Bahr O, Weise L, Harter PN, Hattingen E. MR perfusion in and around the contrast-enhancement of primary CNS lymphomas. J Neurooncol. 2013; 114:127–134.15. Toh CH, Wei KC, Chang CN, Ng SH, Wong HF. Differentiation of primary central nervous system lymphomas and glioblastomas: comparisons of diagnostic performance of dynamic susceptibility contrast-enhanced perfusion MR imaging without and with contrast-leakage correction. AJNR Am J Neuroradiol. 2013; 34:1145–1149.16. Nakajima S, Okada T, Yamamoto A, et al. Differentiation between primary central nervous system lymphoma and glioblastoma: a comparative study of parameters derived from dynamic susceptibility contrast-enhanced perfusion-weighted MRI. Clin Radiol. 2015; 70:1393–1399.17. Nakajima S, Okada T, Yamamoto A, et al. Primary central nervous system lymphoma and glioblastoma: differentiation using dynamic susceptibility-contrast perfusion-weighted imaging, diffusion-weighted imaging, and (18)F-fluorodeoxyglucose positron emission tomography. Clin Imaging. 2015; 39:390–395.18. Xing Z, You RX, Li J, Liu Y, Cao DR. Differentiation of primary central nervous system lymphomas from high-grade gliomas by rCBV and percentage of signal intensity recovery derived from dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Clin Neuroradiol. 2014; 24:329–336.19. Hakyemez B, Erdogan C, Bolca N, Yildirim N, Gokalp G, Parlak M. Evaluation of different cerebral mass lesions by perfusion-weighted MR imaging. J Magn Reson Imaging. 2006; 24:817–824.20. Cho SK, Na DG, Ryoo JW, et al. Perfusion MR imaging: clinical utility for the differential diagnosis of various brain tumors. Korean J Radiol. 2002; 3:171–179.21. Liao W, Liu Y, Wang X, et al. Differentiation of primary central nervous system lymphoma and high-grade glioma with dynamic susceptibility contrast-enhanced perfusion magnetic resonance imaging. Acta Radiol. 2009; 50:217–225.22. Jung SC, Choi SH, Yeom JA, et al. Cerebral blood volume analysis in glioblastomas using dynamic susceptibility contrast-enhanced perfusion MRI: a comparison of manual and semiautomatic segmentation methods. PLoS One. 2013; 8:e69323.23. Heye T, Merkle EM, Reiner CS, et al. Reproducibility of dynamic contrast-enhanced MR imaging. Part II. Comparison of intra- and interobserver variability with manual region of interest placement versus semiautomatic lesion segmentation and histogram analysis. Radiology. 2013; 266:812–821.24. Wetzel SG, Cha S, Johnson G, et al. Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology. 2002; 224:797–803.25. Emblem KE, Nedregaard B, Nome T, et al. Glioma grading by using histogram analysis of blood volume heterogeneity from MR-derived cerebral blood volume maps. Radiology. 2008; 247:808–817.26. Ma JH, Kim HS, Rim NJ, Kim SH, Cho KG. Differentiation among glioblastoma multiforme, solitary metastatic tumor, and lymphoma using whole-tumor histogram analysis of the normalized cerebral blood volume in enhancing and perienhancing lesions. AJNR Am J Neuroradiol. 2010; 31:1699–1706.27. Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med. 1996; 36:715–725.28. Rosen BR, Belliveau JW, Vevea JM, Brady TJ. Perfusion imaging with NMR contrast agents. Magn Reson Med. 1990; 14:249–265.29. Bjornerud A. The ICE software package: direct co-registration of anatomical and functional datasets using DICOM image geometry information. Proc Hum Brain Mapp. 2003; 19:1018.30. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–845.31. Wesseling P, Ruiter DJ, Burger PC. Angiogenesis in brain tumors; pathobiological and clinical aspects. J Neurooncol. 1997; 32:253–265.32. Bauknecht HC, Romano VC, Rogalla P, et al. Intra- and interobserver variability of linear and volumetric measurements of brain metastases using contrast-enhanced magnetic resonance imaging. Invest Radiol. 2010; 45:49–56.33. Kim H, Choi SH, Kim JH, et al. Gliomas: application of cumulative histogram analysis of normalized cerebral blood volume on 3 T MRI to tumor grading. PLoS One. 2013; 8:e63462.34. Odland A, Server A, Saxhaug C, et al. Volumetric glioma quantification: comparison of manual and semi-automatic tumor segmentation for the quantification of tumor growth. Acta Radiol. 2015; 56:1396–1403.35. Ryoo I, Choi SH, Kim JH, et al. Cerebral blood volume calculated by dynamic susceptibility contrast-enhanced perfusion MR imaging: preliminary correlation study with glioblastoma genetic profiles. PLoS One. 2013; 8:e71704.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Measurement of Apparent Diffusion Coefficient Values from Diffusion-Weighted MRI: A Comparison of Manual and Semiautomatic Segmentation Methods
- MR Finding of Primary Renal Lymphoma: A Case Report
- Comparative Study of Dynamic Susceptibility Contrast Perfusion MR Images between Warthin's Tumor and Malignant Parotid Tumors
- Dynamic Contrast-Enhanced MRI and Its Applications in Various Central Nervous System Diseases
- Perfusion Imaging of the Brain Using Z-Score and Dynamic Images Obtained by Subtracting Images from before and after Contrast Injection