J Korean Med Sci.
2017 May;32(5):825-829. 10.3346/jkms.2017.32.5.825.
Reference Values for the Revised Anti-Müllerian Hormone Generation II Assay: Infertile Population-based Study
- Affiliations
-
- 1Hamchoon Women's Clinic, Seoul, Korea.
- 2Medical Research Collaborating Center, Seoul National University Bundang Hospital, Seongnam, Korea.
- 3Department of Obstetrics and Gynecology, Seoul National University Bundang Hospital, Seongnam, Korea.
- 4Samkwang Medical Laboratories, Seoul, Korea.
- 5Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea. seokhyun@snu.ac.kr
- KMID: 2375084
- DOI: http://doi.org/10.3346/jkms.2017.32.5.825
Abstract
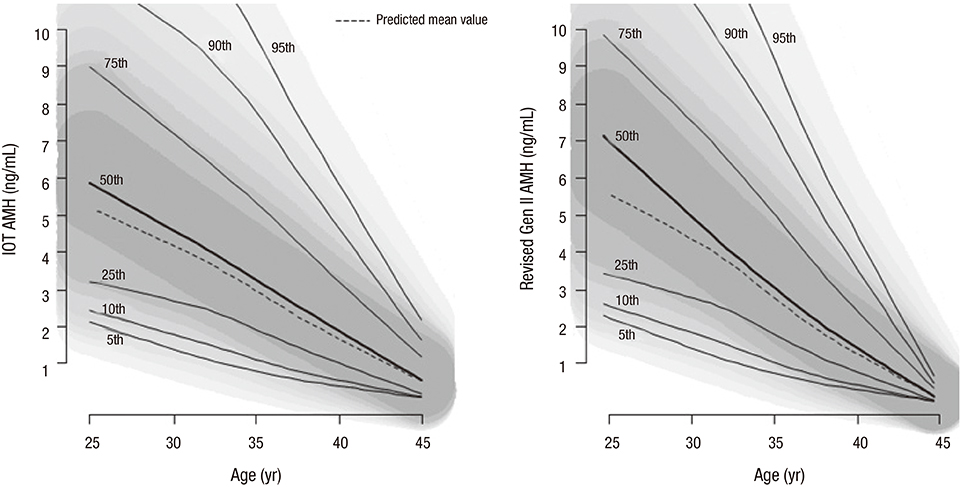
- Anti-Müllerian hormone (AMH) is now accepted as an important clinical marker of ovarian reserve and is increasingly measured as an initial evaluation at infertility clinics. The aim of this study was to establish reference values for the revised second generation (Gen II) assay using population-based data. In this population-based cohort study, AMH data from unselected infertile women aged 25-45 years from June 2013 to June 2014 (n = 15,801) were collected. The AMH values were measured using the revised Gen II assay. We established and validated 5 AMH-age regression models. Based on the optimal AMH-age model, reference values and centile charts were obtained. The quadratic model (log AMH = 0.410 × age − 0.008 × age²âˆ’ 3.791) was the most appropriate for describing the age-dependent decrease in AMH measured using the revised Gen II assay. This is the largest population-based study to establish age-specific reference values of AMH using the revised Gen II assay. These reference values may provide more specific information regarding the ovarian reserve estimation of infertile women.
MeSH Terms
Figure
Cited by 3 articles
-
정상 월경을 가진 한국 여성의 항뮬러관호르몬의 연령별 참고치: 시간에 따른 추세 및 임상적 적합성 (2015-2021년)
Hee-Jeong Kim, Jin Young Park, Mi-Soon Han
Lab Med Online. 2022;12(1):46-52. doi: 10.47429/lmo.2022.12.1.46.Age-specific Reference Intervals for Anti-Müllerian Hormone
Haeil Park
Ann Lab Med. 2022;42(6):617-618. doi: 10.3343/alm.2022.42.6.617.Age Group-specific Reference Intervals for the Elecsys Anti-Müllerian Hormone Assay in Healthy Korean Women: a Nationwide Population-based Study
Misuk Ji, Kwang-Rae Kim, Hyun-Ki Kim, Woochang Lee, Yeo-Min Yun, Sail Chun, Won-Ki Min
Ann Lab Med. 2022;42(6):621-629. doi: 10.3343/alm.2022.42.6.621.
Reference
-
1. Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, Griesinger G, Kelsey TW, La Marca A, Lambalk C, et al. The physiology and clinical utility of anti-Müllerian hormone in women. Hum Reprod Update. 2014; 20:370–385.2. Puntmann VO. How-to guide on biomarkers: biomarker definitions, validation and applications with examples from cardiovascular disease. Postgrad Med J. 2009; 85:538–545.3. Kumar A, Kalra B, Patel A, McDavid L, Roudebush WE. Development of a second generation anti-Müllerian hormone (AMH) ELISA. J Immunol Methods. 2010; 362:51–59.4. Li HW, Ng EH, Wong BP, Anderson RA, Ho PC, Yeung WS. Correlation between three assay systems for anti-Müllerian hormone (AMH) determination. J Assist Reprod Genet. 2012; 29:1443–1446.5. Rustamov O, Smith A, Roberts SA, Yates AP, Fitzgerald C, Krishnan M, Nardo LG, Pemberton PW. Anti-Müllerian hormone: poor assay reproducibility in a large cohort of subjects suggests sample instability. Hum Reprod. 2012; 27:3085–3091.6. Han X, McShane M, Sahertian R, White C, Ledger W. Pre-mixing serum samples with assay buffer is a prerequisite for reproducible anti-Müllerian hormone measurement using the Beckman Coulter Gen II assay. Hum Reprod. 2014; 29:1042–1048.7. Nelson SM, Iliodromiti S, Fleming R, Anderson R, McConnachie A, Messow CM. Reference range for the antimüllerian hormone Generation II assay: a population study of 10,984 women, with comparison to the established diagnostics systems laboratory nomogram. Fertil Steril. 2014; 101:523–529.8. Medicines and Healthcare products Regulatory Agency (GB). Urgent field safety notice-FSN 20434-3 AMH Gen II ELISA (REF A79765), 2 July 2013 [Internet]. accessed on 15 June 2014. Available at http://webarchive.nationalarchives.gov.uk/20141205150130/http://mhra.gov.uk/home/groups/fsn/documents/fieldsafetynotice/con297532.pdf.9. Ceriotti F, Hinzmann R, Panteghini M. Reference intervals: the way forward. Ann Clin Biochem. 2009; 46:8–17.10. Solberg HE, PetitClerc C. International Federation of Clinical Chemistry (IFCC), Scientific Committee, Clinical Section, Expert Panel on Theory of Reference Values. Approved recommendation (1988) on the theory of reference values. Part 3. Preparation of individuals and collection of specimens for the production of reference values. J Clin Chem Clin Biochem. 1988; 26:593–598.11. Lee JY, Jee BC, Lee JR, Kim CH, Park T, Yeon BR, Seo SY, Lee WD, Suh CS, Kim SH. Age-related distributions of anti-Müllerian hormone level and anti-Müllerian hormone models. Acta Obstet Gynecol Scand. 2012; 91:970–975.12. Silverwood RJ, Cole TJ. Statistical methods for constructing gestational age-related reference intervals and centile charts for fetal size. Ultrasound Obstet Gynecol. 2007; 29:6–13.13. Bonellie S, Chalmers J, Gray R, Greer I, Jarvis S, Williams C. Centile charts for birthweight for gestational age for Scottish singleton births. BMC Pregnancy Childbirth. 2008; 8:5–15.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Age-Specific Reference Values of Anti-Müllerian Hormone in Korean Women with Normal Menstruation: Time Trend and Clinical Suitability (2015–2021)
- Serum Anti-Müllerian Hormone Levels in Precocious Puberty Girls according to Stage of GnRH Agonist Treatment
- The expression of Müllerian inhibiting substance/anti-Müllerian hormone type II receptor in myoma and adenomyosis
- Measurement of serum anti-Müllerian hormone by revised Gen II or automated assay: Reproducibility under various blood/serum storage conditions
- Age-related distribution of anti-Mullerian hormone levels in 2,879 Korean women with regular menstruation