J Breast Cancer.
2015 Jun;18(2):126-133. 10.4048/jbc.2015.18.2.126.
Recombinant Human Granulocyte Colony-Stimulating Factor Promotes Preinvasive and Invasive Estrogen Receptor-Positive Tumor Development in MMTV-erbB2 Mice
- Affiliations
-
- 1Department of Oncology, Cancer Institute, First Affiliated Hospital of Henan University of Science and Technology, Luoyang, China. fengxiaoshan2014@163.com
- KMID: 2374527
- DOI: http://doi.org/10.4048/jbc.2015.18.2.126
Abstract
- PURPOSE
We investigated whether recombinant human granulocyte colony-stimulating factor (rhG-CSF) could promote the development of preinvasive and invasive breast cancer in mouse mammary tumor virus (MMTV-erbB2) mice with estrogen receptor-positive tumors.
METHODS
MMTV-erbB2 mice were randomly divided into three experimental groups with 20 mice in each group. MMTV-erbB2 mice were treated with daily subcutaneous injections of vehicle or rhG-CSF (low-rhG-CSF group, rhG-CSF 0.125 microg; vehicle-rhG-CSF group, normal saline 0.25 microg; and high-rhG-CSF group, rhG-CSF 0.25 microg) at 3 months of age. Cellular and molecular mechanisms of G-CSF action in mammary glands were investigated via immunohistochemistry and reverse transcription polymerase chain reaction.
RESULTS
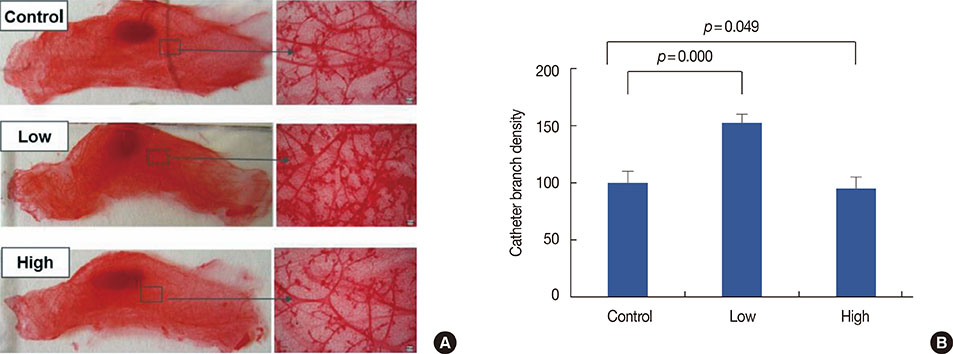
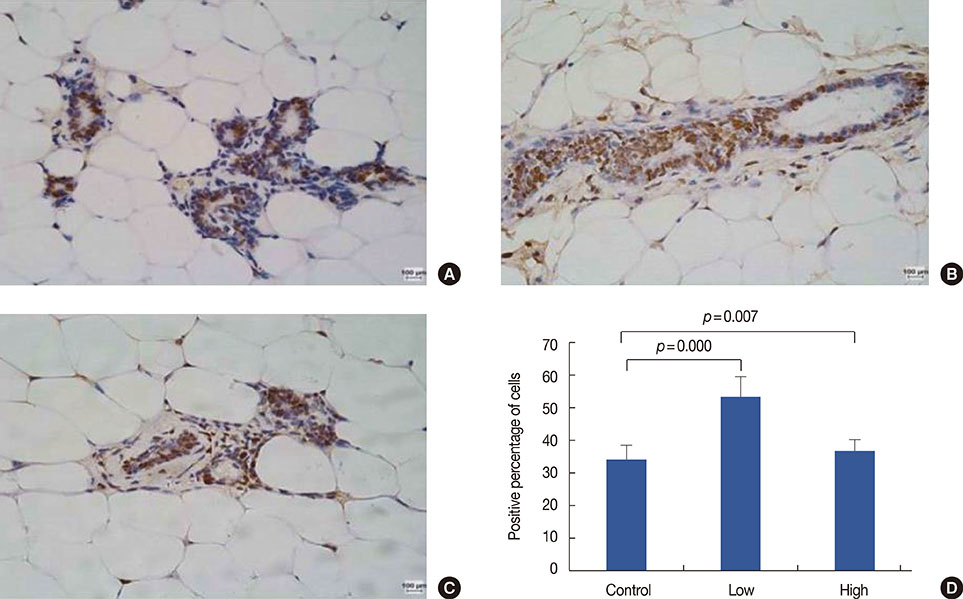
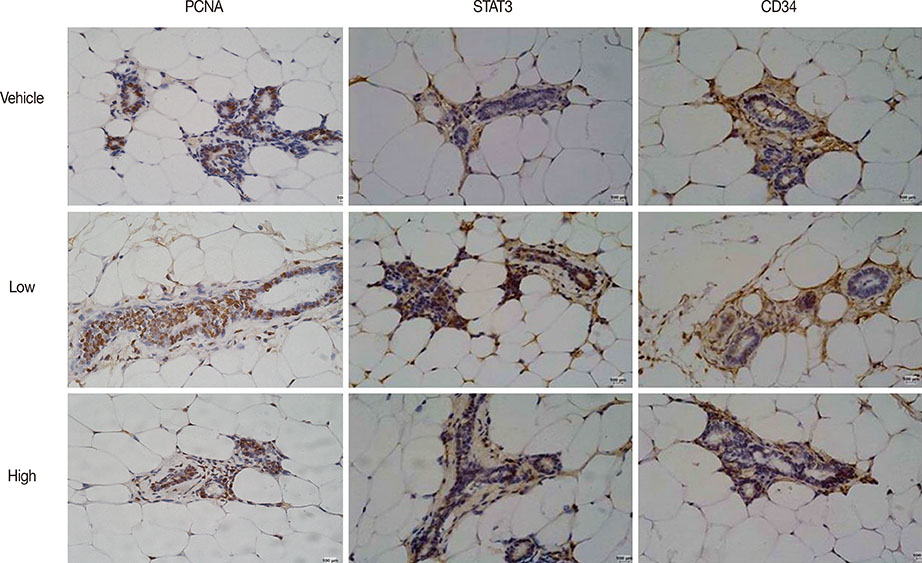
Low, but not high, rhG-CSF doses significantly accelerated mammary tumorigenesis in MMTV-erbB2 mice. Short-term treatment with rhG-CSF could significantly promote the development of preinvasive mammary lesions. The cancer prevention effect was associated with reduced expression of proliferating cell nuclear antigen, cluster of differentiation 34, and signal transducers and activators of transcription 3 in mammary glands by >80%.
CONCLUSION
We found that G-CSF was regulated by rhG-CSF both in vitro and in vivo. Identification of G-CSF genes helped us further understand the mechanism by which G-CSF promotes cancer. Low doses of rhG-CSF could significantly increase tumor latency and increase tumor multiplicity and burden. Moreover, rhG-CSF effectively promotes development of both malignant and premalignant mammary lesions in MMTV-erbB2 mice.
MeSH Terms
-
Animals
Breast Neoplasms
Carcinogenesis
Cell Proliferation
Estrogens*
Granulocyte Colony-Stimulating Factor*
Humans
Immunohistochemistry
Injections, Subcutaneous
Mammary Glands, Human
Mammary Tumor Virus, Mouse
Mice*
Polymerase Chain Reaction
Proliferating Cell Nuclear Antigen
Reverse Transcription
Transducers
Estrogens
Granulocyte Colony-Stimulating Factor
Proliferating Cell Nuclear Antigen
Figure
Reference
-
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009; 59:225–249.
Article2. Hirbe AC, Uluçkan O, Morgan EA, Eagleton MC, Prior JL, Piwnica-Worms D, et al. Granulocyte colony-stimulating factor enhances bone tumor growth in mice in an osteoclast-dependent manner. Blood. 2007; 109:3424–3431.
Article3. Mattei S, Colombo MP, Melani C, Silvani A, Parmiani G, Herlyn M. Expression of cytokine/growth factors and their receptors in human melanoma and melanocytes. Int J Cancer. 1994; 56:853–857.
Article4. Natori T, Sata M, Washida M, Hirata Y, Nagai R, Makuuchi M. G-CSF stimulates angiogenesis and promotes tumor growth: potential contribution of bone marrow-derived endothelial progenitor cells. Biochem Biophys Res Commun. 2002; 297:1058–1061.
Article5. Noda I, Fujieda S, Ohtsubo T, Tsuzuki H, Tanaka N, Sunaga H, et al. Granulocyte-colony-stimulating factor enhances invasive potential of human head-and-neck-carcinoma cell lines. Int J Cancer. 1999; 80:78–84.
Article6. Obermueller E, Vosseler S, Fusenig NE, Mueller MM. Cooperative autocrine and paracrine functions of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor in the progression of skin carcinoma cells. Cancer Res. 2004; 64:7801–7812.
Article7. Savarese TM, Mitchell K, McQuain C, Campbell CL, Guardiani R, Wuu J, et al. Coexpression of granulocyte colony stimulating factor and its receptor in primary ovarian carcinomas. Cancer Lett. 2001; 162:105–115.
Article8. Yang X, Liu F, Xu Z, Chen C, Wu X, Li G, et al. Expression of granulocyte colony stimulating factor receptor in human colorectal cancer. Postgrad Med J. 2005; 81:333–337.
Article9. Morales-Arias J, Meyers PA, Bolontrade MF, Rodriguez N, Zhou Z, Reddy K, et al. Expression of granulocyte-colony-stimulating factor and its receptor in human Ewing sarcoma cells and patient tumor specimens: potential consequences of granulocyte-colony-stimulating factor administration. Cancer. 2007; 110:1568–1577.
Article10. Ichiishi E, Yoshikawa T, Kogawa T, Yoshida N, Kondo M. Possible paracrine growth of adenocarcinoma of the stomach induced by granulocyte colony stimulating factor produced by squamous cell carcinoma of the oesophagus. Gut. 2000; 46:432–434.
Article11. Tachibana M, Miyakawa A, Nakashima J, Murai M, Nakamura K, Kubo A, et al. Autocrine growth promotion by multiple hematopoietic growth factors in the established renal cell carcinoma line KU-19-20. Cell Tissue Res. 2000; 301:353–367.
Article12. Bussolino F, Wang JM, Defilippi P, Turrini F, Sanavio F, Edgell CJ, et al. Granulocyte- and granulocyte-macrophage-colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature. 1989; 337:471–473.
Article13. Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989; 339:27–30.
Article14. Nicola NA. Hemopoietic cell growth factors and their receptors. Annu Rev Biochem. 1989; 58:45–77.
Article15. Pébusque MJ, Lopez M, Torres H, Carotti A, Guilbert L, Mannoni P. Growth response of human myeloid leukemia cells to colony-stimulating factors. Exp Hematol. 1988; 16:360–366.16. Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991; 78:2791–2808.
Article17. Avalos BR. Molecular analysis of the granulocyte colony-stimulating factor receptor. Blood. 1996; 88:761–777.
Article18. Bashey A, Healy L, Marshall CJ. Proliferative but not nonproliferative responses to granulocyte colony-stimulating factor are associated with rapid activation of the p21ras/MAP kinase signalling pathway. Blood. 1994; 83:949–957.
Article19. Rausch O, Marshall CJ. Tyrosine 763 of the murine granulocyte colony-stimulating factor receptor mediates Ras-dependent activation of the JNK/SAPK mitogen-activated protein kinase pathway. Mol Cell Biol. 1997; 17:1170–1179.
Article20. Nicholson SE, Novak U, Ziegler SF, Layton JE. Distinct regions of the granulocyte colony-stimulating factor receptor are required for tyrosine phosphorylation of the signaling molecules JAK2, Stat3, and p42, p44MAPK. Blood. 1995; 86:3698–3704.
Article21. Pandya K, Meeke K, Clementz AG, Rogowski A, Roberts J, Miele L, et al. Targeting both Notch and ErbB-2 signalling pathways is required for prevention of ErbB-2-positive breast tumour recurrence. Br J Cancer. 2011; 105:796–806.
Article22. Kröger N, Sonnenberg S, Cortes-Dericks L, Freiberger P, Mollnau H, Zander AR. Kinetics of G-CSF and CD34+ cell mobilization after once or twice daily stimulation with rHu granulocyte-stimulating factor (lenograstim) in healthy volunteers: an intraindividual crossover study. Transfusion. 2004; 44:104–110.
Article23. Hernández-Bernal F, García-García I, González-Delgado CA, Valenzuela-Silva C, Soto-Hernández R, Ducongé J, et al. Bioequivalence of two recombinant granulocyte colony-stimulating factor formulations in healthy male volunteers. Biopharm Drug Dispos. 2005; 26:151–159.
Article24. Hutchinson JN, Muller WJ. Transgenic mouse models of human breast cancer. Oncogene. 2000; 19:6130–6137.
Article25. Beckmann MW, Niederacher D, Schnürch HG, Gusterson BA, Bender HG. Multistep carcinogenesis of breast cancer and tumour heterogeneity. J Mol Med (Berl). 1997; 75:429–439.
Article26. Kosanke S, Edgerton SM, Moore D 2nd, Yang X, Mason T, Alvarez K, et al. Mammary tumor heterogeneity in wt-ErbB-2 transgenic mice. Comp Med. 2004; 54:280–287.27. Kumar J, Fraser FW, Riley C, Ahmed N, McCulloch DR, Ward AC. Granulocyte colony-stimulating factor receptor signalling via Janus kinase 2/signal transducer and activator of transcription 3 in ovarian cancer. Br J Cancer. 2014; 110:133–145.
Article28. Moon HW, Kim TY, Oh BR, Hwang SM, Kwon J, Ku JL, et al. Effects of granulocyte-colony stimulating factor and the expression of its receptor on various malignant cells. Korean J Hematol. 2012; 47:219–224.
Article29. Panopoulos AD, Watowich SS. Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and 'emergency' hematopoiesis. Cytokine. 2008; 42:277–288.
Article30. Park HB, Yang JH, Chung KH. Characterization of the cytokine profile of platelet rich plasma (PRP) and PRP-induced cell proliferation and migration: upregulation of matrix metalloproteinase-1 and -9 in HaCaT cells. Korean J Hematol. 2011; 46:265–273.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two cases of congenital agranulocytosis treated with recombinant human granulocyte colony-stimulating factor
- Sweet Syndrome in a Child with Aplastic Anemia after Receiving Recombinant Granulocyte Colony-stimulating Factor
- A Case of Methimazole-induced Pancytopenia: Successful Treatment with Recombinant Human Granulocyte Colony-stimulating Factor
- The effect of granulocyte colony-stimulating factor in chemotherapy of acute myelogenous leukemia
- Preliminary study of antithymocyte or antilymphocyte globulin, cyclosporine-A and recombinant human granulocyte macrophage colony stimulating factors for patients with aplastic anemia