J Korean Med Assoc.
2017 Mar;60(3):251-256. 10.5124/jkma.2017.60.3.251.
Pathogenesis of allergic conjunctivitis and trends in its treatment
- Affiliations
-
- 1Department of Ophthalmology, Incheon St. Mary's Hospital, The Catholic University of Korea, College of Medicine, Incheon, Korea. leoanzel@catholic.ac.kr
- KMID: 2374386
- DOI: http://doi.org/10.5124/jkma.2017.60.3.251
Abstract
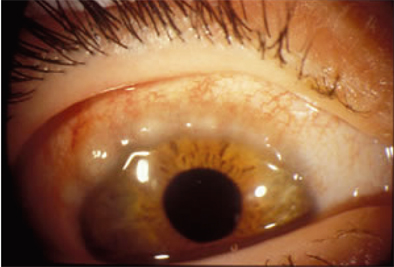
- Allergic conjunctivitis is characterized by specific immunologic responses known as type 1 hypersensitivity, resulting in corneal and conjunctival inflammation. Histamine plays an important role in the pathophysiologic mechanism of allergic conjunctivitis. Five subtypes of allergic conjunctivitis have been defined according to specific signs and symptoms: seasonal allergic conjunctivitis, perennial allergic conjunctivitis, vernal keratoconjunctivitis, atopic keratoconjunctivitis and giant papillary conjunctivitis. Above all, avoidance of the offending antigen is the primary behavioral modification used to treat all types of allergic conjunctivitis. However, this may be difficult for practical reasons; therefore, a range of medical treatment options, such as topical antihistamines, mast cell stabilizers, non-steroidal anti-inflammatory drugs, and corticosteroids are prescribed in clinical practice.
MeSH Terms
Figure
Reference
-
1. Chigbu DI. The pathophysiology of ocular allergy: a review. Cont Lens Anterior Eye. 2009; 32:3–15.
Article2. Wong AH, Barg SS, Leung AK. Seasonal and perennial aller-gic conjunctivitis. Recent Pat Inflamm Allergy Drug Discov. 2014; 8:139–153.
Article3. Mantelli F, Calder VL, Bonini S. The anti-inflammatory effects of therapies for ocular allergy. J Ocul Pharmacol Ther. 2013; 29:786–793.
Article4. Ridolo E, Montagni M, Caminati M, Senna G, Incorvaia C, Canonica GW. Emerging drugs for allergic conjunctivitis. Expert Opin Emerg Drugs. 2014; 19:291–302.
Article5. Ono SJ, Lane K. Comparison of effects of alcaftadine and olopatadine on conjunctival epithelium and eosinophil recruitment in a murine model of allergic conjunctivitis. Drug Des Devel Ther. 2011; 5:77–84.6. Bonini S, Micera A, Iovieno A, Lambiase A, Bonini S. Expres-sion of Toll-like receptors in healthy and allergic conjunctiva. Ophthalmology. 2005; 112:1528.
Article7. Torkildsen G, Shedden A. The safety and efficacy of alcafta-dine 0.25% ophthalmic solution for the prevention of itching associated with allergic conjunctivitis. Curr Med Res Opin. 2011; 27:623–631.
Article8. Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009; 106:11330–11335.
Article9. Marson CM. Targeting the histamine H4 receptor. Chem Rev. 2011; 111:7121–7156.10. Bhatt HG, Agrawal YK, Raval HG, Manna K, Desai PR. Histamine H4 receptor: a novel therapeutic target for immune and allergic responses. Mini Rev Med Chem. 2010; 10:1293–1308.
Article11. Cook EB, Stahl JL, Sedgwick JB, Barney NP, Graziano FM. The promotion of eosinophil degranulation and adhesion to conjunctival epithelial cells by IgE-activated conjunctival mast cells. Ann Allergy Asthma Immunol. 2004; 92:65–72.
Article12. Kosina-Hagyo K, Veres A, Fodor E, Mezei G, Csakany B, Nemeth J. Tear film function in patients with seasonal allergic conjunctivitis outside the pollen season. Int Arch Allergy Immunol. 2012; 157:81–88.
Article13. Juniper EF, Guyatt GH, Ferrie PJ, King DR. Sodium cromo-glycate eye drops: regular versus “as needed” use in the treatment of seasonal allergic conjunctivitis. J Allergy Clin Immunol. 1994; 94:36–43.
Article14. Chatterjee S, Agrawal D. Tacrolimus in corticosteroid-refrac-tory vernal keratoconjunctivitis. Cornea. 2016; 35:1444–1448.
Article15. Westland T, Patryn EK, Nieuwendaal CP, van der Meulen IJ, Mourits MP, Lapid-Gortzak R. Vernal shield ulcers treated with frequently installed topical cyclosporine 0.05% eyedrops. Int Ophthalmol. 2017; 01. 24. DOI: 10.1007/s10792-016-0424-z. [Epub].
Article16. Cornish KS, Gregory ME, Ramaesh K. Systemic cyclosporin A in severe atopic keratoconjunctivitis. Eur J Ophthalmol. 2010; 20:844–851.
Article17. Chigbu DI, Coyne AM. Update and clinical utility of alcafta-dine ophthalmic solution 0.25% in the treatment of allergic conjunctivitis. Clin Ophthalmol. 2015; 9:1215–1225.18. Erdinest N, Solomon A. Topical immunomodulators in the management of allergic eye diseases. Curr Opin Allergy Clin Immunol. 2014; 14:457–463.
Article19. Barot RK, Shitole SC, Bhagat N, Patil D, Sawant P, Patil K. Therapeutic effect of 0.1% tacrolimus eye ointment in allergic ocular diseases. J Clin Diagn Res. 2016; 10:NC05–NC09.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis and pharmacological management of allergic conjunctivitis
- Bilateral Conjunctival Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma Misdiagnosed as Allergic Conjunctivitis
- Clinical Effect of Naaxia(R) Therapy on Allergic Conjunctivitis
- The Relationship between Allergic Conjunctivitis due to Brimonidine and Systemic Allergic Disease in Glaucoma Patients
- The Expression of Adhesion Molecules in Experimental Allergic and Chemical Conjunctivitis