Yonsei Med J.
2017 Jan;58(1):217-225. 10.3349/ymj.2017.58.1.217.
Cyclosporine Sparing Effect of Enteric-Coated Mycophenolate Sodium in De Novo Kidney Transplantation
- Affiliations
-
- 1Department of Surgery, Ajou University School of Medicine, Suwon, Korea.
- 2Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. jbpakrmd@gmail.com
- 3Department of Surgery, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Surgery, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2374209
- DOI: http://doi.org/10.3349/ymj.2017.58.1.217
Abstract
- PURPOSE
The increased tolerability of enteric-coated mycophenolate sodium (EC-MPS), compared to mycophenolate mofetil, among kidney transplant recipients has the potential to facilitate cyclosporine (CsA) minimization. Therefore, a prospective trial to determine the optimum EC-MPS dose in CsA-based immunosuppression regimens is necessary.
MATERIALS AND METHODS
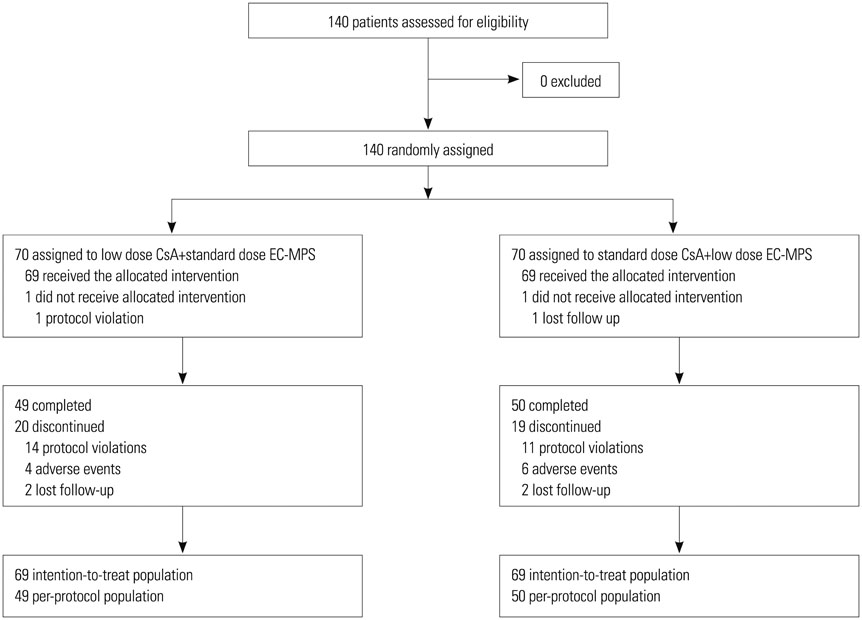
A comparative, parallel, randomized, open-label study was performed for 140 patients from four centers to compare the efficacy and tolerability of low dose CsA with standard dose EC-MPS (the investigational group) versus standard dose CsA with low dose EC-MPS (the control group) for six months in de novo kidney transplant recipients. Graft function, the incidence of efficacy failure [biopsy-confirmed acute rejection (BCAR), death, graft loss, loss to follow-up], and adverse events were compared.
RESULTS
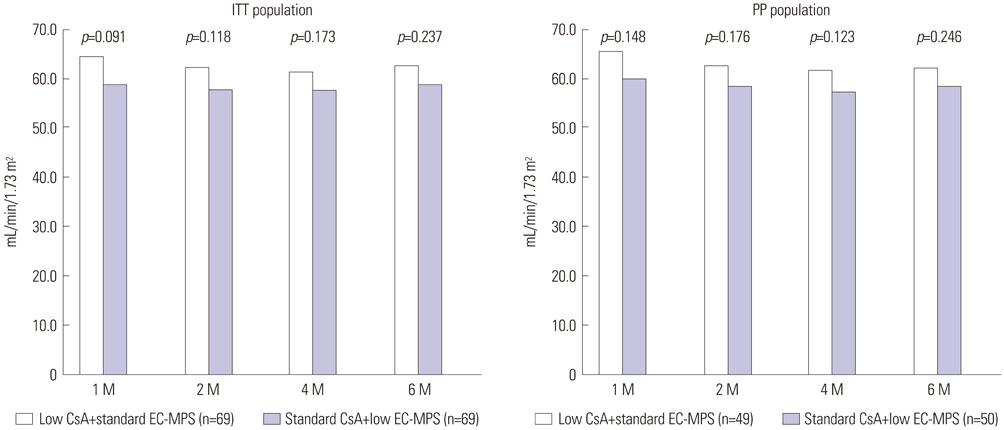
The mean estimated glomerular filtration rate (eGFR) of the investigational group at six months post-transplantation was non-inferior to that of the control group (confidence interval between 57.3 mL/min/1.73m² and 67.4 mL/min/1.73 m², p<0.001). One graft loss was reported in the control group, and no patient deaths were reported in either group. The incidence of BCAR of the investigational group was 8.7%, compared to 18.8% in the control group (p=0.137), during the study period. There were no significant differences (p>0.05) in the incidence of discontinuations and serious adverse events (SAE) between the groups.
CONCLUSION
CsA minimization using a standard dose of EC-MPS kept the incidence of acute rejection and additional risks as low as conventional immunosuppression and provided therapeutic equivalence in terms of renal graft function and safety issues.
MeSH Terms
-
Adult
Aged
Cyclosporine/*administration & dosage
Female
Graft Rejection/*prevention & control
Humans
Immunosuppressive Agents/*administration & dosage
Incidence
Kidney Transplantation
Male
Middle Aged
Mycophenolic Acid/*administration & dosage
Prospective Studies
Tablets, Enteric-Coated
Time Factors
Cyclosporine
Immunosuppressive Agents
Mycophenolic Acid
Tablets, Enteric-Coated
Figure
Reference
-
1. Tantravahi J, Womer KL, Kaplan B. Why hasn't eliminating acute rejection improved graft survival? Annu Rev Med. 2007; 58:369–385.
Article2. Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004; 4:378–383.
Article3. Meier-Kriesche HU, Schold JD, Kaplan B. Long-term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant. 2004; 4:1289–1295.
Article4. Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Chapman JR, Allen RD. Calcineurin inhibitor nephrotoxicity: longitudinal assessment by protocol histology. Transplantation. 2004; 78:557–565.
Article5. Bolin P, Tanriover B, Zibari GB, Lynn ML, Pirsch JD, Chan L, et al. Improvement in 3-month patient-reported gastrointestinal symptoms after conversion from mycophenolate mofetil to entericcoated mycophenolate sodium in renal transplant patients. Transplantation. 2007; 84:1443–1451.
Article6. Dalal P, Grafals M, Chhabra D, Gallon L. Mycophenolate mofetil: safety and efficacy in the prophylaxis of acute kidney transplantation rejection. Ther Clin Risk Manag. 2009; 5:139–149.7. Budde K, Glander P, Krämer BK, Fischer W, Hoffmann U, Bauer S, et al. Conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium in maintenance renal transplant recipients receiving tacrolimus: clinical, pharmacokinetic, and pharmacodynamic outcomes. Transplantation. 2007; 83:417–424.
Article8. Salvadori M, Bertoni E, Budde K, Holzer H, Civati G, Lien B, et al. Superior efficacy of enteric-coated mycophenolate vs mycophenolate mofetil in de novo transplant recipients: pooled analysis. Transplant Proc. 2010; 42:1325–1328.
Article9. Cooper M, Salvadori M, Budde K, Oppenheimer F, Sollinger H, Zeier M, et al. Enteric-coated mycophenolate sodium immunosuppression in renal transplant patients: efficacy and dosing. Transplant Rev (Orlando). 2012; 26:233–240.
Article10. Langone A, Shihab F, Pankewycz O, Doria C, Wiland A, McCague K, et al. Long-term dosing patterns of enteric-coated mycophenolate sodium or mycophenolate mofetil with tacrolimus after renal transplantation. Clin Transplant. 2014; 28:961–967.
Article11. Oh CK, Huh KH, Lee JS, Cho HR, Kim YS. Safety and efficacy of conversion from twice-daily tacrolimus to once-daily tacrolimus one month after transplantation: randomized controlled trial in adult renal transplantation. Yonsei Med J. 2014; 55:1341–1347.
Article12. Ekberg H, Grinyó J, Nashan B, Vanrenterghem Y, Vincenti F, Voulgari A, et al. Cyclosporine sparing with mycophenolate mofetil, daclizumab and corticosteroids in renal allograft recipients: the CAESAR Study. Am J Transplant. 2007; 7:560–570.
Article13. Hernández D, Miquel R, Porrini E, Fernández A, González-Posada JM, Hortal L, et al. Randomized controlled study comparing reduced calcineurin inhibitors exposure versus standard cyclosporine-based immunosuppression. Transplantation. 2007; 84:706–714.
Article14. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999; 130:461–470.
Article15. Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999; 55:713–723.
Article16. Ekberg H, Tedesco-Silva H, Demirbas A, Vütko S, Nashan B, Gürkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007; 357:2562–2575.
Article17. Etienne I, Toupance O, Bénichou J, Thierry A, Al Najjar A, Hurault de Ligny B, et al. A 50% reduction in cyclosporine exposure in stable renal transplant recipients: renal function benefits. Nephrol Dial Transplant. 2010; 25:3096–3106.
Article18. Sollinger H. Enteric-coated mycophenolate sodium: therapeutic equivalence to mycophenolate mofetil in de novo renal transplant patients. Transplant Proc. 2004; 36:2 Suppl. 517S–520S.
Article19. Ortega F, Sánchez-Fructuoso A, Cruzado JM, Gómez-Alamillo JC, Alarcón A, Pallardó L, et al. Gastrointestinal quality of life improvement of renal transplant recipients converted from mycophenolate mofetil to enteric-coated mycophenolate sodium drugs or agents: mycophenolate mofetil and enteric-coated mycophenolate sodium. Transplantation. 2011; 92:426–432.
Article20. Panarelli NC. Drug-induced injury in the gastrointestinal tract. Semin Diagn Pathol. 2014; 31:165–175.
Article21. Cai L, Zeng F, Liu B, Wei L, Chen Z, Jiang J. A single-centre, open-label, prospective study of an initially short-term intensified dosing regimen of enteric-coated mycophenolate sodium with reduced cyclosporine A exposure in Chinese live-donor kidney transplant recipients. Int J Clin Pract Suppl. 2014; (181):23–30.
Article22. Arns W, Sommerer C, Glander P, Ariatabar T, Porstner M, May C, et al. A randomized trial of intensified vs. standard dosing for enteric-coated mycophenolate sodium in de novo kidney transplant recipients: results at 1 year. Clin Nephrol. 2013; 79:421–431.
Article23. Chadban S, Eris J, Russ G, Campbell S, Chapman J, Pussell B, et al. Enteric-coated mycophenolate sodium in combination with full dose or reduced dose cyclosporine, basiliximab and corticosteroids in Australian de novo kidney transplant patients. Nephrology (Carlton). 2013; 18:63–70.
Article24. Budde K, Bosmans JL, Sennesael J, Zeier M, Pisarski P, Schütz M, et al. Reduced-exposure cyclosporine is safe and efficacious in de novo renal transplant recipients treated with enteric-coated mycophenolic acid and basiliximab. Clin Nephrol. 2007; 67:164–175.
Article25. Cibrik D, Meier-Kriesche HU, Bresnahan B, Wu YM, Klintmalm G, Kew CE, et al. Renal function with cyclosporine C2 monitoring, enteric-coated mycophenolate sodium and basiliximab: a 12-month randomized trial in renal transplant recipients. Clin Transplant. 2007; 21:192–201.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Safety and Efficacy of the Early Introduction of Everolimus (Certican(R)) with Low Dose of Cyclosporine in de Novo Kidney Recipients after 1 Month of Transplantation (Preliminary Results)
- Mycophenolate Mofetil and Prednisolone as Maintenance Therapy in Hemolytic Uremic Syndrome after Kidney Transplantation
- Improved Gastrointestinal Symptoms and Quality of Life after Conversion from Mycophenolate Mofetil to Enteric-Coated Mycophenolate Sodium in Renal Transplant Patients Receiving Tacrolimus
- The impact of omeprazole on mycophenolate pharmacokinetics in kidney transplant recipients
- Association of human leukocyte antigen homozygosity and de novo malignancies in kidney transplant recipients