Yonsei Med J.
2016 Jul;57(4):905-914. 10.3349/ymj.2016.57.4.905.
Assessment of Denosumab in Korean Postmenopausal Women with Osteoporosis: Randomized, Double-Blind, Placebo-Controlled Trial with Open-Label Extension
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea.
- 3Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, Korea.
- 4Department of Endocrinology and Metabolism, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea.
- 5Department of Endocrinology and Metabolism, Pusan National University Hospital, Busan, Korea.
- 6Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 7Department of Family Medicine, Cheil General Hospital, College of Medicine, Kwandong University, Seoul, Korea.
- 8Department of Orthopedic Surgery, Kyungpook National University Hospital, Daegu, Korea.
- 9GlaxoSmithKline, Seoul, Korea.
- 10GlaxoSmithKline, Collegeville, PA, USA. barbara.g.kravitz@gsk.com
- KMID: 2374122
- DOI: http://doi.org/10.3349/ymj.2016.57.4.905
Abstract
- PURPOSE
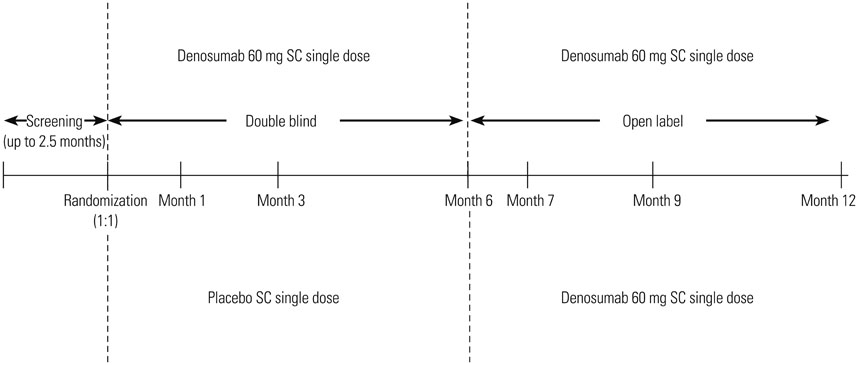
The efficacy and safety of denosumab was compared with placebo in Korean postmenopausal women with osteoporosis in this phase III study.
MATERIALS AND METHODS
Women aged 60 to 90 years with a T-score of <-2.5 and ≥-4.0 at the lumbar spine or total hip were randomized to a single 60 mg subcutaneous dose of denosumab or placebo for the 6-month double-blind phase. Eligible subjects entered the 6-month open-label extension phase and received a single dose of denosumab 60 mg.
RESULTS
Baseline demographics were similar in the 62 denosumab- and 64 placebo-treated subjects who completed the double-blind phase. Treatment favored denosumab over placebo for the primary endpoint {mean percent change from baseline in lumbar spine bone mineral density (BMD) at Month 6 [3.2% (95% confidence interval 2.1%, 4.4%; p<0.0001)]}; and secondary endpoints (mean percent change from baseline in lumbar spine BMD at Month 1, total hip, femoral neck, and trochanter BMD at Months 1 and 6, and median percent change from baseline in bone turnover markers at Months 1, 3, and 6). Endpoint improvements were sustained over 12 months in the open-label extension (n=119). There were no new or unexpected safety signals.
CONCLUSION
Denosumab was well tolerated and effective in increasing BMD and decreasing bone turnover markers over a 12-month period in Korean postmenopausal women. The findings of this study demonstrate that denosumab has beneficial effects on the measures of osteoporosis in Korean postmenopausal women.
Keyword
MeSH Terms
-
Aged
Aged, 80 and over
*Asian Continental Ancestry Group
Bone Density
Bone Density Conservation Agents/*therapeutic use
Denosumab/*therapeutic use
Double-Blind Method
Female
Femur
Femur Neck
Humans
Lumbar Vertebrae
Middle Aged
Osteoporosis, Postmenopausal/*drug therapy/*ethnology
Postmenopause
Republic of Korea
Bone Density Conservation Agents
Denosumab
Figure
Cited by 2 articles
-
Pharmacologic treatment of osteoporosis
Yong-Ki Min
J Korean Med Assoc. 2016;59(11):847-856. doi: 10.5124/jkma.2016.59.11.847.Real-World Safety and Effectiveness of Denosumab in Patients with Osteoporosis: A Prospective, Observational Study in South Korea
Yumie Rhee, Dong-Gune Chang, Jeonghoon Ha, Sooa Kim, Yusun Lee, Euna Jo, Jung-Min Koh
Endocrinol Metab. 2022;37(3):497-505. doi: 10.3803/EnM.2022.1427.
Reference
-
1. Choi YJ, Oh HJ, Kim DJ, Lee Y, Chung YS. The prevalence of osteoporosis in Korean adults aged 50 years or older and the higher diagnosis rates in women who were beneficiaries of a national screening program: the Korea National Health and Nutrition Examination Survey 2008-2009. J Bone Miner Res. 2012; 27:1879–1886.
Article2. Kim JW, Jeon YJ, Baek DH, Kim TN, Chang JS. Percentage of the population at high risk of osteoporotic fracture in South Korea: analysis of the 2010 Fifth Korean National Health and Nutrition Examination survey data. Osteoporos Int. 2014; 25:1313–1319.
Article3. Mithal A, Ebeling P, Kyer CS. The Asia-pacific regional audit: epidemiology, costs & burden of osteoporosis in 2013s. International Osteoporosis Foundation;accessed on 2014 January 23. Available at: http://www.iofbonehealth.org/sites/default/files/media/PDFs/Regional%20Audits/2013-Asia_Pacific_Audit_0_0.pdf.4. Kostenuik PJ. Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr Opin Pharmacol. 2005; 5:618–625.
Article5. Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009; 361:756–765.
Article6. Papapoulos S, Chapurlat R, Libanati C, Brandi ML, Brown JP, Czerwiński E, et al. Five years of denosumab exposure in women with postmenopausal osteoporosis: results from the first two years of the FREEDOM extension. J Bone Miner Res. 2012; 27:694–701.
Article7. Bone HG, Chapurlat R, Brandi ML, Brown JP, Czerwinski E, Krieg MA, et al. The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab. 2013; 98:4483–4492.
Article8. Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001; 344:1434–1441.
Article9. Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000; 343:604–610.
Article10. World Health Organization. WHO scientific group on the assessment of osteoporosis at primary health care level: summary meeting report; Brussels, Belgium, 5-7 May 2004. accessed on 2013 December 9. Available at: http://www.who.int/chp/topics/Osteoporosis.pdf.11. Pitale S, Thomas M, Rathi G, Deshmukh V, Kumar P, Reddy S, et al. Effects of denosumab in postmenopausal women with osteoporosis from India (abstract). Indian J Endocrinol Metab. 2013; 17:Suppl1. S373–S394.12. Nakamura T, Matsumoto T, Sugimoto T, Shiraki M. Dose-response study of denosumab on bone mineral density and bone turnover markers in Japanese postmenopausal women with osteoporosis. Osteoporos Int. 2012; 23:1131–1140.
Article13. Chesnut CH 3rd, Silverman S, Andriano K, Genant H, Gimona A, Harris S, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med. 2000; 109:267–276.
Article14. Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997; 337:670–676.
Article15. Nakamura T, Matsumoto T, Sugimoto T, Hosoi T, Miki T, Gorai I, et al. Clinical Trials Express: fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: denosumab fracture intervention randomized placebo controlled trial (DIRECT). J Clin Endocrinol Metab. 2014; 99:2599–2607.
Article16. Lim SK, Kung AW, Sompongse S, Soontrapa S, Tsai KS. Vitamin D inadequacy in postmenopausal women in Eastern Asia. Curr Med Res Opin. 2008; 24:99–106.
Article17. Delmas PD. Markers of bone turnover for monitoring treatment of osteoporosis with antiresorptive drugs. Osteoporos Int. 2000; 11:Suppl 6. S66–S76.
Article18. Iwamoto J, Takeda T, Sato Y, Uzawa M. Determinants of one-year response of lumbar bone mineral density to alendronate treatment in elderly Japanese women with osteoporosis. Yonsei Med J. 2004; 45:676–682.
Article19. Lau EMC, Sambrook P, Seeman E, Leong KH, Leung PC, Delmas P. Guidelines for diagnosing, prevention and treatment of osteoporosis in Asia. APLAR J Rheumatol. 2006; 9:24–36.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Update on Denosumab Treatment in Postmenopausal Women with Osteoporosis
- Denosumab for the treatment of osteoporosis
- Effect of multivitamin on serum 25-hydroxy vitamin D level in postmenopausal women: A randomized, double-blind, placebo-controlled trial
- Denosumab (RANKL Inhibitor): A Potent Anti-Resorptive Agent
- The Effects of Oral Calcifediol in Postmenopausal Women with Osteopenia and Osteoporosis