Yonsei Med J.
2016 May;57(3):735-740. 10.3349/ymj.2016.57.3.735.
The Relationship between HIF-2α and VEGF with Radiographic Severity in the Primary Osteoarthritic Knee
- Affiliations
-
- 1Department of Orthopedics, Renmin Hospital of Wuhan University, Wuhan, China. zhoujianlin2005@sina.com
- KMID: 2374095
- DOI: http://doi.org/10.3349/ymj.2016.57.3.735
Abstract
- PURPOSE
The aim of this study was to determine the relationship of hypoxia-inducible factor-2 (HIF-2α) and vascular endothelial growth factor (VEGF) with radiographic severity in primary osteoarthritis (OA) of the knee. Expression of these two factors in cartilage samples from OA knee joints was examined at mRNA and protein levels.
MATERIALS AND METHODS
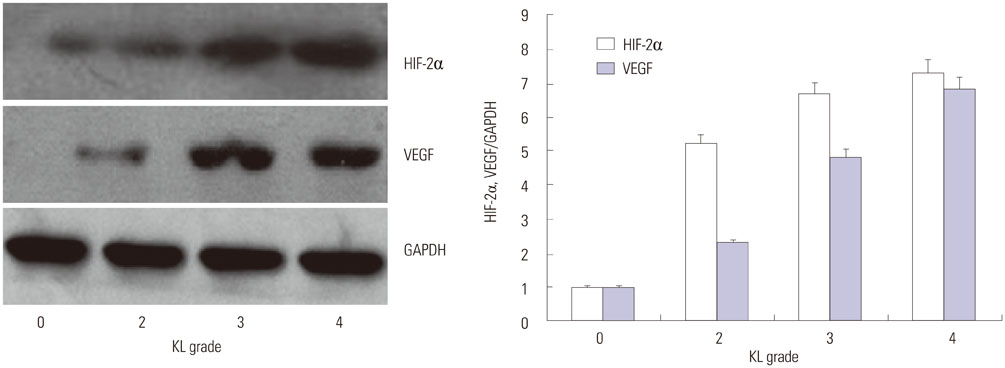
Knee joints were examined using plain radiographs, and OA severity was assessed using the Kellgren and Lawrence (KL) grading system. Specimens were collected from 29 patients (31 knees) who underwent total knee replacement because of severe medial OA of the knee (KL grades 3 and 4), 16 patients who underwent knee arthroscopy (KL grade 2), and 5 patients with traumatic knees (KL grade 0). HIF-2α and VEGF expression was quantified by real-time polymerase chain reaction and western blotting.
RESULTS
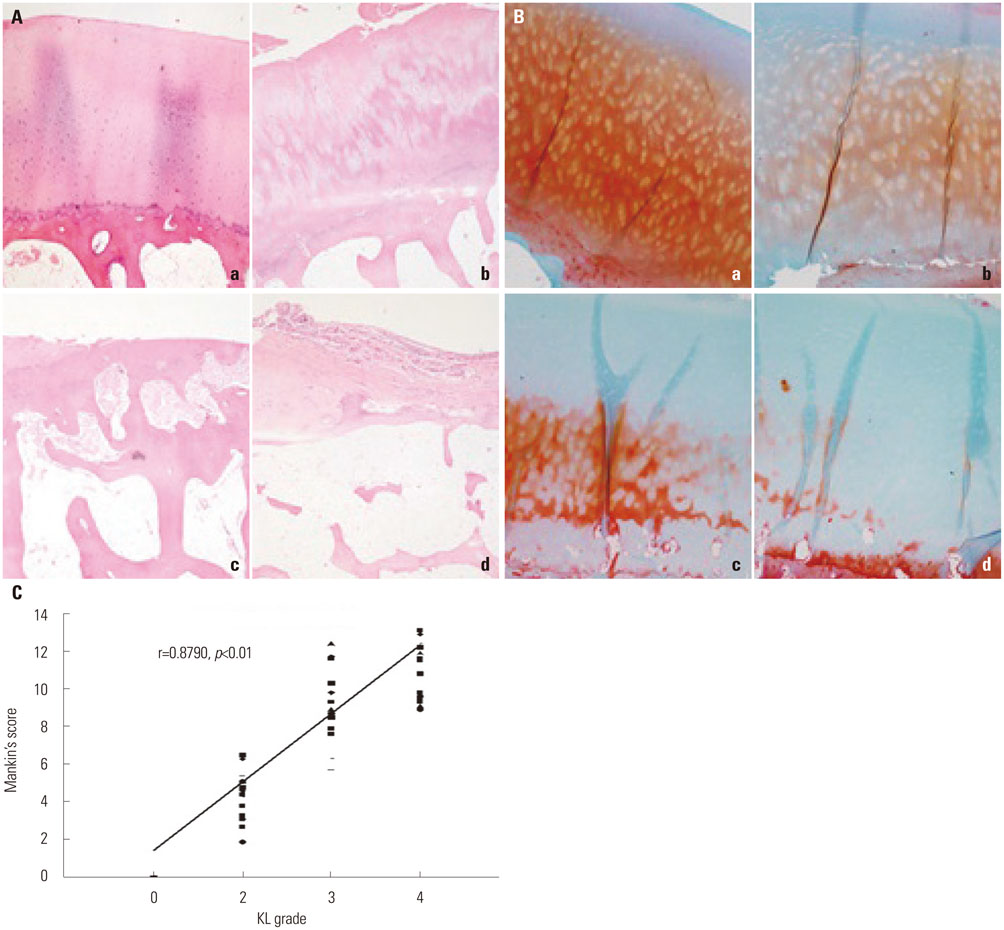
Cartilage degeneration correlated with the radiographic severity grade. OA severity, determined using the Mankin scale, correlated positively with the KL grade (r=0.8790, p<0.01), and HIF-2α and VEGF levels with the radiographic severity of knee OA (r=0.7001, p<0.05; r=0.6647, p<0.05).
CONCLUSION
In OA cartilage, HIF-2α and VEGF mRNA and protein levels were significantly and positively correlated. The expression of both factors correlated positively with the KL grade. HIF-2α and VEGF, therefore, may serve as biochemical markers as well as potential therapeutic targets in knee OA.
Keyword
MeSH Terms
-
Adult
Aged
Arthroplasty, Replacement, Knee
Arthroscopy
Basic Helix-Loop-Helix Transcription Factors/*metabolism
Biomarkers/*blood
Cartilage/*metabolism
Female
Humans
Knee Joint/*diagnostic imaging
Male
Middle Aged
Osteoarthritis, Knee/*blood/diagnostic imaging/physiopathology
RNA, Messenger
Radiography
Real-Time Polymerase Chain Reaction
Severity of Illness Index
Vascular Endothelial Growth Factor A/*metabolism
Basic Helix-Loop-Helix Transcription Factors
Biomarkers
RNA, Messenger
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Hunter DJ, Felson DT. Osteoarthritis. BMJ. 2006; 332:639–642.
Article2. Sharma L, Kapoor D. Epidemiology of osteoarthritis. In : Moskowitz RW, Altman RD, Hochberg MC, Buckwalter JA, Goldberg VM, editors. Osteoarthritis: Diagnosis and Medical/Surgical Management. 4th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins;2007. p. 3–26.3. Yang S, Kim J, Ryu JH, Oh H, Chun CH, Kim BJ, et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010; 16:687–693.
Article4. Pfander D, KÖrtje D, Zimmermann R, Weseloh G, Kirsch T, Gesslein M, et al. Vascular endothelial growth factor in articular cartilage of healthy and osteoarthritic human knee joints. Ann Rheum Dis. 2001; 60:1070–1073.
Article5. Saito T, Fukai A, Mabuchi A, Ikeda T, Yano F, Ohba S, et al. Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development. Nat Med. 2010; 16:678–686.
Article6. Enomoto H, Inoki I, Komiya K, Shiomi T, Ikeda E, Obata K, et al. Vascular endothelial growth factor isoforms and their receptors are expressed in human osteoarthritic cartilage. Am J Pathol. 2003; 162:171–181.
Article7. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957; 16:494–502.
Article8. Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971; 53:523–537.
Article9. Saito T, Kawaguchi H. HIF-2α as a possible therapeutic target of osteoarthritis. Osteoarthritis Cartilage. 2010; 18:1552–1556.
Article10. Saetan N, Honsawek S, Tanavalee A, Yuktanandana P, Meknavin S, Ngarmukos S, et al. Relationship of plasma and synovial fluid vascular endothelial growth factor with radiographic severity in primary knee osteoarthritis. Int Orthop. 2014; 38:1099–1104.
Article11. Ryu JH, Shin Y, Huh YH, Yang S, Chun CH, Chun JS. Hypoxia-inducible factor-2α regulates Fas-mediated chondrocyte apoptosis during osteoarthritic cartilage destruction. Cell Death Differ. 2012; 19:440–450.
Article12. Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999; 5:623–628.
Article13. Horner A, Bord S, Kelsall AW, Coleman N, Compston JE. Tie2 ligands angiopoietin-1 and angiopoietin-2 are coexpressed with vascular endothelial cell growth factor in growing human bone. Bone. 2001; 28:65–71.
Article14. Pufe T, Petersen W, Tillmann B, Mentlein R. The splice variants VEGF121 and VEGF189 of the angiogenic peptide vascular endothelial growth factor are expressed in osteoarthritic cartilage. Arthritis Rheum. 2001; 44:1082–1088.
Article15. Zhou JL, Liu SQ, Qiu B, Hu QJ, Ming JH, Peng H. Effects of hyaluronan on vascular endothelial growth factor and receptor-2 expression in a rabbit osteoarthritis model. J Orthop Sci. 2009; 14:313–319.
Article16. Chen XY, Hao YR, Wang Z, Zhou JL, Jia QX, Qiu B. The effect of vascular endothelial growth factor on aggrecan and type II collagen expression in rat articular chondrocytes. Rheumatol Int. 2012; 32:3359–3364.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Relationship between Expression of Hypoxia Inducible Factor-1alpha or Vascular Endothelial Growth Factor and Histopathological Characteristics in Human Transitional Bladder Cancer
- The Relationship between Expression of Hypoxia Inducible Factor-1alpha or Vascular Endothelial Growth Factor and Histopathological Characteristics in Human Renal Cell Carcinoma
- Immunohistochemical Expression and Prognostic Value of VEGF, HIF-1alpha, EGFR in Non-Small Cell Lung Cancer
- Hypoxia-inducible factor: role in cell survival in superoxide dismutase overexpressing mice after neonatal hypoxia-ischemia
- Hypoxia-Inducible Factor-1 Alpha Stabilization in Human Macrophages during Leishmania major Infection Is Impaired by Parasite Virulence