Ann Lab Med.
2016 Sep;36(5):450-456. 10.3343/alm.2016.36.5.450.
Analytical and Clinical Validation of Six Commercial Middle East Respiratory Syndrome Coronavirus RNA Detection Kits Based on Real-Time Reverse-Transcription PCR
- Affiliations
-
- 1Department of Laboratory Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea. mnkim@amc.seoul.kr
- 2Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea.
- 3Department of Laboratory Medicine, Hallym University College of Medicine, Hallym University Kangdong Sacred Heart Hospital, Seoul, Korea.
- 4Department of Laboratory Medicine, Inje University, Sanggye Paik Hospital, Seoul, Korea.
- KMID: 2373575
- DOI: http://doi.org/10.3343/alm.2016.36.5.450
Abstract
- BACKGROUND
During the 2015 outbreak of Middle East Respiratory Syndrome coronavirus (MERS-CoV), six different commercial MERS-CoV RNA detection kits based on real-time reverse-transcription polymerase chain reaction (rRT-PCR) were available in Korea. We performed analytical and clinical validations of these kits.
METHODS
PowerChek (Kogene Biotech, Korea), DiaPlexQ (SolGent, Korea), Anyplex (Seegene, Korea), AccuPower (Bioneer, Korea), LightMix (Roche Molecular Diagnostics, Switzerland), and UltraFast kits (Nanobiosys, Korea) were evaluated. Limits of detection (LOD) with 95% probability values were estimated by testing 16 replicates of upstream of the envelope gene (upE) and open reading frame 1a (ORF1a) RNA transcripts. Specificity was estimated by using 28 nasopharyngeal swabs that were positive for other respiratory viruses. Clinical sensitivity was evaluated by using 18 lower respiratory specimens. The sensitivity test panel and the high inhibition panel were composed of nine specimens each, including eight and six specimens that were positive for MERS-CoV, respectively.
RESULTS
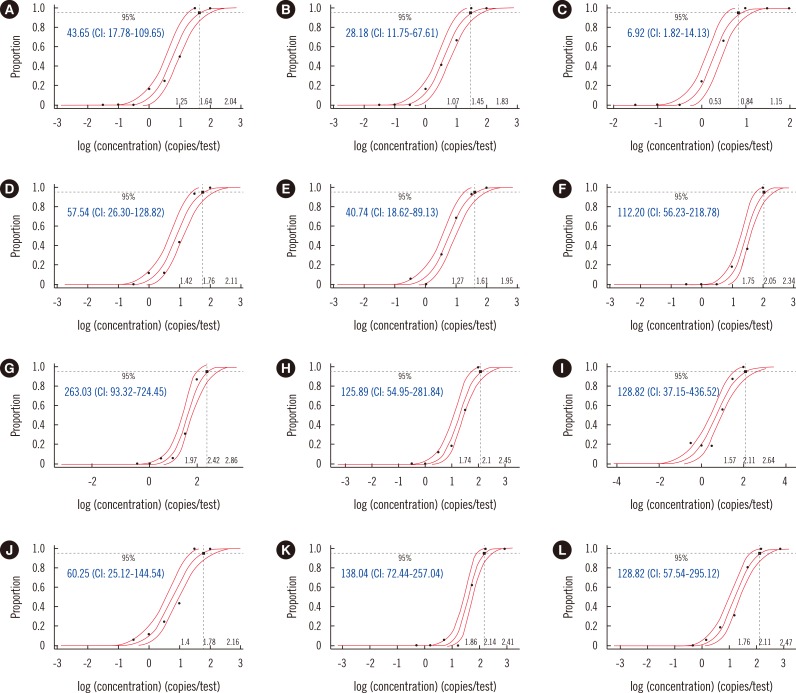
The LODs for upE ranged from 21.88 to 263.03 copies/reaction, and those for ORF1a ranged from 6.92 to 128.82 copies/reaction. No cross-reactivity with other respiratory viruses was found. All six kits correctly identified 8 of 8 (100%) positive clinical specimens. Based on results from the high inhibition panel, PowerChek and AccuPower were the least sensitive to the presence of PCR inhibition.
CONCLUSIONS
The overall sensitivity and specificity of all six assay systems were sufficient for diagnosing MERS-CoV infection. However, the analytical sensitivity and detection ability in specimens with PCR inhibition could be improved with the use of appropriate internal controls.
Keyword
MeSH Terms
-
Coronavirus Infections/diagnosis/virology
Humans
Middle East Respiratory Syndrome Coronavirus/*genetics/isolation & purification
Nasopharynx/virology
Open Reading Frames/genetics
RNA, Viral/*analysis/metabolism
Reagent Kits, Diagnostic
*Real-Time Polymerase Chain Reaction
Viral Envelope Proteins/genetics
RNA, Viral
Reagent Kits, Diagnostic
Viral Envelope Proteins
Figure
Cited by 1 articles
-
Quality of Ribonucleic Acid Extraction for Real-Time Reverse Transcription-PCR (rRT-PCR) of SARS-CoV-2: Importance of Internal Control Monitoring
Yeon Joo Lee, Youngeun Lim, Kyu Wha Hur, Heungsup Sung, Mi-Na Kim
Ann Lab Med. 2020;40(6):490-492. doi: 10.3343/alm.2020.40.6.490.
Reference
-
1. Cowling BJ, Park M, Fang VJ, Wu P, Leung GM, Wu JT. Preliminary epidemiological assessment of MERS-CoV outbreak in South Korea, May to June 2015. Euro Surveill. 2015; 20:7–13. PMID: 26132767.2. Park HY, Lee EJ, Ryu YW, Kim Y, Kim H, Lee H, et al. Epidemiological investigation of MERS-CoV spread in a single hospital in South Korea, May to June 2015. Euro Surveill. 2015; 20:1–6. PMID: 26132766.3. WHO. Laboratory testing for Middle East Respiratory Syndrome Coronavirus 2014. Updated on Sep 2014. http://www.who.int/csr/disease/coronavirus_infections/WHO_interim_recommendations_lab_detection_MERSCoV_092014.pdf.4. Center for Disease Control and Prevention. Laboratory testing for Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Updated on Jun 2014. http://www.cdc.gov/coronavirus/mers/lab/lab-testing.html.5. Lu X, Whitaker B, Sakthivel SK, Kamili S, Rose LE, Lowe L, et al. Real-time reverse transcription-PCR assay panel for Middle East respiratory syndrome coronavirus. J Clin Microbiol. 2014; 52:67–75. PMID: 24153118.6. Center for Disease Control and Prevention. Information for Laboratories. Updated on Dec 2015. http://www.cdc.gov/coronavirus/mers/lab/index.html.7. Corman VM, Müller MA, Costabel U, Timm J, Binger T, Meyer B, et al. Assays for laboratory confirmation of novel human coronavirus (hCoV-EMC) infections. Euro Surveill. 2012; 17:pii: 20334.8. Corman VM, Eckerle I, Bleicker T, Zaki A, Landt O, Eschbach-Bludau M, et al. Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Euro Surveill. 2012; 17:pii: 20285.9. Corman VM, Ölschläger S, Wendtner CM, Drexler JF, Hess M, Drosten C. Performance and clinical validation of the RealStar MERS-CoV Kit for detection of Middle East respiratory syndrome coronavirus RNA. J Clin Virol. 2014; 60:168–171. PMID: 24726679.10. Madej RM, Davis J, Holden MJ, Kwang S, Labourier E, Schneider GJ. International standards and reference materials for quantitative molecular infectious disease testing. J Mol Diagn. 2010; 12:133–143. PMID: 20075208.11. Kraft CS, Armstrong WS, Caliendo AM. Interpreting quantitative cytomegalovirus DNA testing: understanding the laboratory perspective. Clin Infect Dis. 2012; 54:1793–1797. PMID: 22412060.12. Schnepf N, Scieux C, Resche-Riggon M, Feghoul L, Xhaard A, Gallien S, et al. Fully automated quantification of cytomegalovirus (CMV) in whole blood with the new sensitive Abbott RealTime CMV assay in the era of the CMV international standard. J Clin Microbiol. 2013; 51:2096–2102. PMID: 23616450.13. Cotten M. Preliminary analysis of Middle East respiratory syndrome coronavirus (MERS-CoV) sequences from Korea and China. Updated on Jun 2015. http://virological.org/t/preliminary-analysis-of-middle-east-respiratory-syndrome-coronavirus-mers-cov-sequences-from-korea-and-china/143.14. Clinical and Laboratory Standards Institute. Quantitative molecular methods for infectious disease. Approved guideline-second edition, MM06-A2. Wayne, PA: Clinical and Laboratory Standard Institutete;2010.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of the PowerChek MERS (upE & ORF1a) Real-Time PCR Kit for the Detection of Middle East Respiratory Syndrome Coronavirus RNA
- Laboratory Diagnosis of COVID-19 in Korea
- Comparison of RNA Extraction Kits for Detection of HCV RNA
- Evaluation of Three Multiplex Realtime Reverse Transcription PCR Assays for Simultaneous Detection of SARS-CoV-2, Influenza A/B, and Respiratory Syncytial Virus in Nasopharyngeal Swabs
- Evaluation of a Multiplex Real-time PCR Assay for the Detection of Respiratory Viruses in Clinical Specimens