Ewha Med J.
2021 Jan;44(1):1-10. 10.12771/emj.2021.44.1.1.
Laboratory Diagnosis of COVID-19 in Korea
- Affiliations
-
- 1Department of Laboratory Medicine, Ewha Womans University Seoul Hospital, Seoul, Korea
- KMID: 2512405
- DOI: http://doi.org/10.12771/emj.2021.44.1.1
Abstract
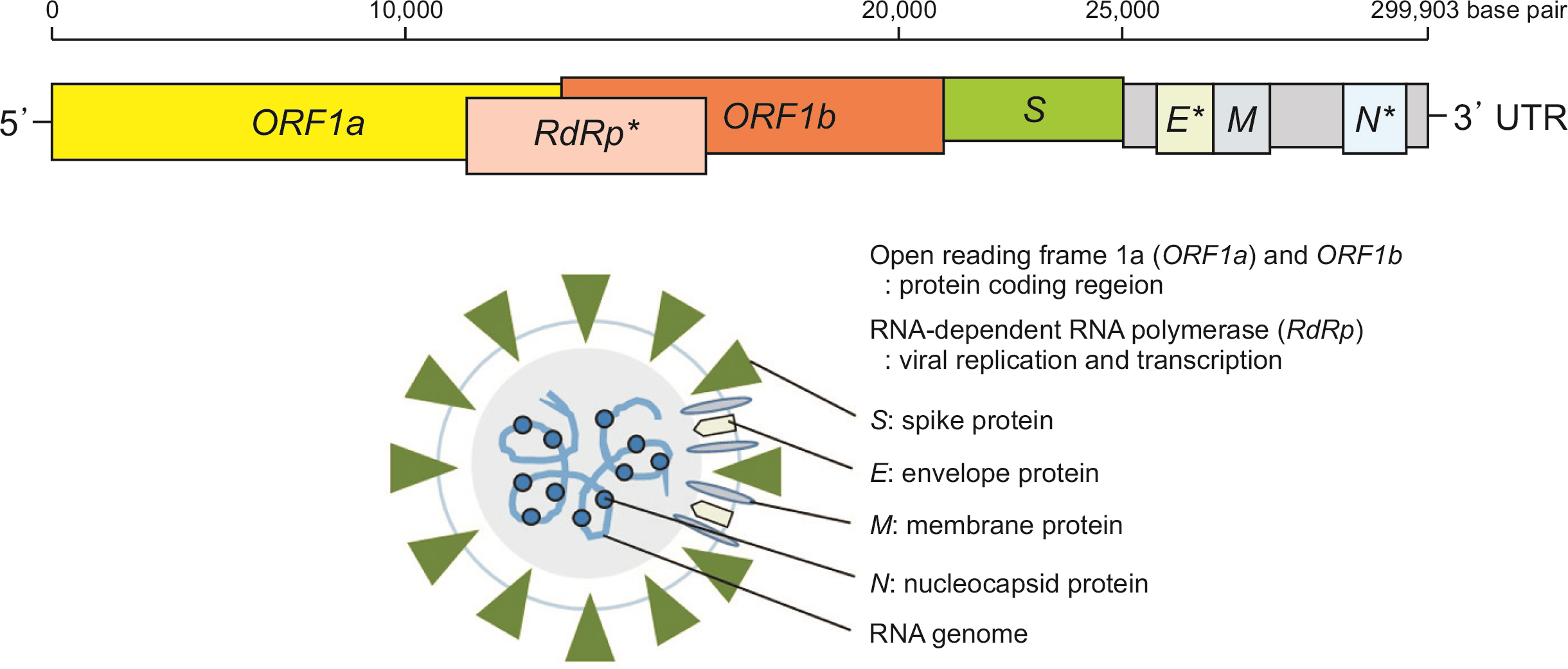
- Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), is a type of human coronavirus that causes severe pneumonia, similar to SARS-CoV-1 and Middle East respiratory syndrome coronavirus. In Korea, the SARS-CoV-2 testing has started quickly from February 2020 to respond to the COVID-19 pandemic. In this article, I would like to introduce the characteristics of coronavirus and PCR test methods that play a large role in COVID-19 quarantine measures. Real-time reverse transcription (RT)-PCR is one of the molecular diagnostic method, and it detect SARS-CoV-2 RNA by amplifying SARS-CoV-2 specific RdRp (RNA-dependent RNA polymerase) gene and E (envelope) gene at the same time. Real-time RT-PCR is currently the most reliable test that confirming COVID-19 and is in use worldwide. Real-time RT-PCR test is recommended for COVID-19 confirmatory diagnosis in Korea, but this test requires dedicated equipment, reagents, experienced technicians and laboratory medicine specialists, and it takes about a few hours to a day to report. Rapid molecular testing results in one to two hours with a simple procedure. Antigen test is less sensitive than real-time RT-PCR and can only be used as a secondary role of diagnosis. As the global COVID-19 pandemic progresses, diagnostic testing guidelines and recommendations may vary and will be updated as scientific evidence and experience of the COVID-19 accumulates.
Figure
Cited by 1 articles
-
Inconsistent Polymerase Chain Reaction Test Results From the Upper And Lower Airways of a Patient Who Underwent Total Laryngectomy During the Incubation Period for Coronavirus Disease
Dong Yun Lee, Myung Jin Ban
Korean J Otorhinolaryngol-Head Neck Surg. 2023;66(7):485-488. doi: 10.3342/kjorl-hns.2022.00808.
Reference
-
1. Ministry of Food and Drug Safety. c2020. Guidelines for the development and review/approval of in vitro diagnostic devices for COVID-19 [Internet]. National Institute of Food and Drug Safety Evaluation;Cheongju (KR): Available from: https://www.nifds.go.kr/brd/m_15/view.do?.seq=12887. cited 2021 Jan 8.2. World Health Organization. c2020. Disease outbreak news: novel coronavirus-Republic of Korea (ex-China) [Internet]. World Health Organization;Geneva (CH): Available from: https://www.who.int/csr/don/21-january-2020-novel-coronavirus-republic-of-korea-ex-china/en/. cited 2021 Jan 8.3. World Health Organization. c2020. Report of the WHO-China Joint Mission on coronavirus disease 2019 (COVID-19) [Internet]. World Health Organization;Geneva (CH): Available from: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf. cited 2021 Jan 8.4. World Health Organization. c2020. Naming the coronavirus disease (COVID-19) and the virus that causes it [Internet]. World Health Organization;Geneva (CH): Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it. cited 2021 Jan 8.5. World Health Organization. c2020. WHO coronavirus disease (COVID-19) dashboard [Internet]. World Health Organization;Geneva (CH): Available from: https://covid19.who.int/table. cited 2021 Jan 8.6. Ministry of Health and Welfare. Coronavirus disease-19, Republic of Korea [Internet]. Ministry of Health and Welfare;Sejong (KR): Available from: http://ncov.mohw.go.kr/. cited 2021 Jan 8.7. Paules CI, Marston HD, Fauci AS. 2020; Coronavirus infections: more than just the common cold. JAMA. 323:707–708. DOI: 10.1001/jama.2020.0757. PMID: 31971553.8. Heo JY. 2020; Clinical and epidemiological characteristics of coronavirus disease 2019 in the early stage of outbreak. Korean J Med. 95:67–73. DOI: 10.3904/kjm.2020.95.2.67.
Article9. Backer JA, Klinkenberg D, Wallinga J. 2020; Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20-28 January 2020. Euro Surveill. 25:2000062. DOI: 10.2807/1560-7917.ES.2020.25.5.2000062. PMID: 32046819. PMCID: PMC7014672.
Article10. Korea Central Disease Control Headquarters. c2020. Information on medical procedures in medical centers for the simultaneous waves of flu and COVID-19 [Internet]. Ministry of Health and Welfare;Sejong (KR): Available from: http://ncov.mohw.go.kr/shBoardView.do?.brdId=2&brdGubun=24&ncvContSeq=4090. cited 2021 Jan 8.11. World Health Organization. c2020. Transmission of SARS-CoV-2: implications for infection prevention precautions [Internet]. World Health Organization;Geneva (CH): Available from: https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions. cited 2021 Jan 8.12. Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. 2020; COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 24:91–98. DOI: 10.1016/j.jare.2020.03.005. PMID: 32257431. PMCID: PMC7113610.13. Ministry of Health and Welfare. c2020. Korea Centers for Disease Control and Prevention begins to develop a novel coronavirus test method [Internet]. Ministry of Health and Welfare;Sejong (KR): Available from: http://www.mohw.go.kr/react/al/sal0301vw.jsp?.PAR_MENU_ID=04&MENU_ID=0403&page=1&CONT_SEQ=352333. cited 2021 Jan 8.14. COVID-19 Diagnosis Test Management Committee. c2020. Guidelines for the laboratory diagnosis of COVID-19 in Korea, 4th edition [Internet]. Korean Society for Laboratory Medicine;Seoul (KR): Available from: http://www.kslm.org/rang_board/list.html?.num=16943&code=covid19_press. cited 2021 Jan 8.15. Feng W, Newbigging AM, Le C, Pang B, Peng H, Cao Y, et al. 2020; Molecular diagnosis of COVID-19: challenges and research needs. Anal Chem. 92:10196–10209. DOI: 10.1021/acs.analchem.0c02060. PMID: 32573207. PMCID: PMC7346719.
Article16. COVID-19 Diagnosis Test Management Committee. c2020. Position of the Korean Society for Laboratory Medicine on COVID-19 test_20201222 [Internet]. Korean Society for Laboratory Medicine;Seoul (KR): Available from: http://www.kslm.org/rang_board/list.html?.num=16964&code=covid19_press. cited 2021 Jan 8.17. Centers for Disease Control and Prevention. c2020. Interim guidelines for collecting, handling, and testing clinical specimens from persons for coronavirus disease 2019 (COVID-19) [Internet]. Centers for Disease Control and Prevention;Atlanta (GA): Available from: https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html. cited 2021 Jan 8.18. Sung H, Kang JO, Lee NY, Lee CK, Kim HS, Lee KM, et al. 2015; Comparison of nasopharyngeal aspirates and nasopharyngeal flocked swabs for respiratory virus detection. Ann Clin Microbiol. 18:119–125. DOI: 10.5145/ACM.2015.18.4.119.
Article19. COVID-19 Diagnosis Test Management Committee. c2020. COVID-19 test Q and A, version 4 [Internet]. Korean Society for Laboratory Medicine;Seoul (KR): Available from: http://www.kslm.org/rang_board/list.html?.num=16811&code=covid19_qna. cited 2021 Jan 8.20. Hong KH, Lee SW, Kim TS, Huh HJ, Lee J, Kim SY, et al. 2020; Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea. Ann Lab Med. 40:351–360. DOI: 10.3343/alm.2020.40.5.351. PMID: 32237288. PMCID: PMC7169629.
Article21. Sung H, Roh KH, Hong KH, Seong MW, Ryoo N, Kim HS, et al. 2020; COVID-19 molecular testing in Korea: practical essentials and answers from experts based on experiences of emergency use authorization assays. Ann Lab Med. 40:439–447. DOI: 10.3343/alm.2020.40.6.439. PMID: 32539299. PMCID: PMC7295959.
Article22. Hur KH, Park K, Lim Y, Jeong YS, Sung H, Kim MN. 2020; Evaluation of four commercial kits for SARS-CoV-2 real-time reverse-transcription polymerase chain reaction approved by emergency-use-authorization in Korea. Front Med (Lausanne). 7:521. DOI: 10.3389/fmed.2020.00521. PMID: 32903503. PMCID: PMC7438443.
Article23. Uhm JS, Ahn JY, Hyun J, Sohn Y, Kim JH, Jeong SJ, et al. 2020; Patterns of viral clearance in the natural course of asymptomatic COVID-19: comparison with symptomatic non-severe COVID-19. Int J Infect Dis. 99:279–285. DOI: 10.1016/j.ijid.2020.07.070. PMID: 32763446. PMCID: PMC7403105.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current laboratory diagnosis of coronavirus disease 2019
- Laboratory Diagnosis and Utilization for COVID-19
- Osteonecrosis following Steroid Therapy in COVID-19 Patients: An Outlook on the Emerging Problem
- The Management of Thyroid Disease in COVID-19 Pandemic
- Assessment and Management of Dysphagia during the COVID-19 Pandemic