Ann Lab Med.
2016 Jul;36(4):291-299. 10.3343/alm.2016.36.4.291.
Screening PCR Versus Sanger Sequencing: Detection of CALR Mutations in Patients With Thrombocytosis
- Affiliations
-
- 1Department of Laboratory Medicine, Gachon University Gil Medical Center, Incheon, Korea. jyahn66@naver.com, pwpark@gilhospital.com
- 2Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea.
- KMID: 2373547
- DOI: http://doi.org/10.3343/alm.2016.36.4.291
Abstract
- BACKGROUND
Mutations in calreticulin (CALR) have been reported to be key markers in the molecular diagnosis of myeloid proliferative neoplasms. In most previous reports, CALR mutations were analyzed by using Sanger sequencing. Here, we report a new, rapid, and convenient system for screening CALR mutations without sequencing.
METHODS
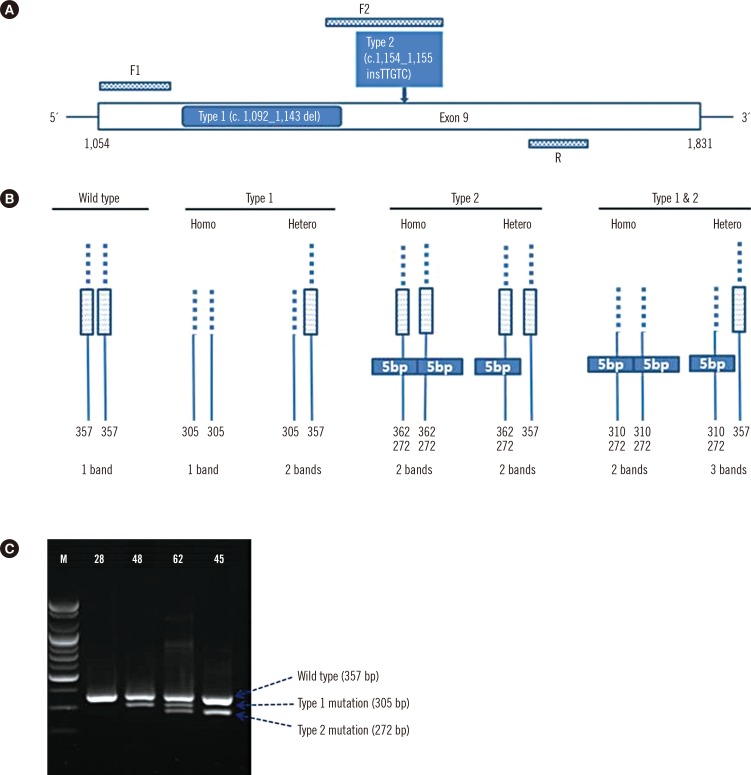
Eighty-three bone marrow samples were obtained from 81 patients with thrombocytosis. PCR primers were designed to detect wild-type CALR (product: 357 bp) and CALR with type 1 (product: 302 bp) and type 2 mutations (product: 272 bp) in one reaction. The results were confirmed by Sanger sequencing and compared with results from fragment analysis.
RESULTS
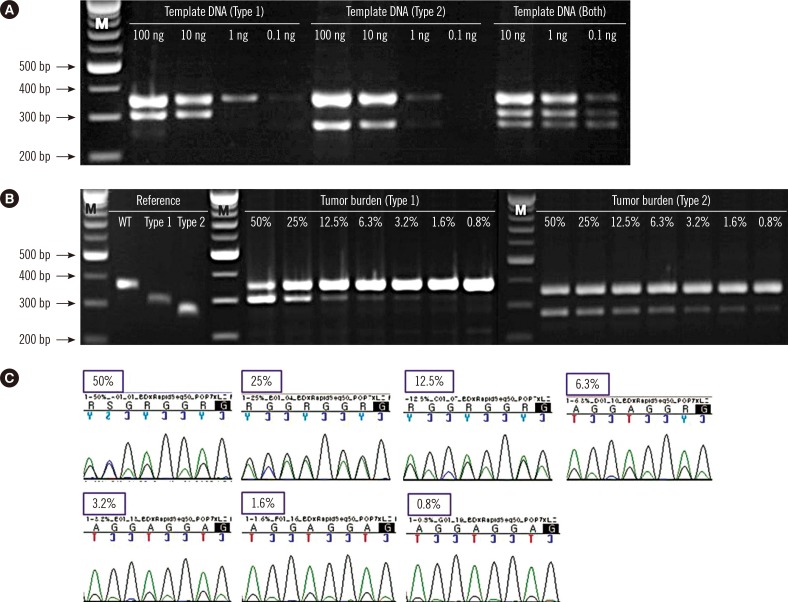
The minimum detection limit of the screening PCR was 10 ng for type 1, 1 ng for type 2, and 0.1 ng for cases with both mutations. CALR type 1 and type 2 mutants were detected with screening PCR with a maximal analytical sensitivity of 3.2% and <0.8%, respectively. The screening PCR detected 94.1% (16/17) of mutation cases and showed concordant results with sequencing in the cases of type 1 and type 2 mutations. Sanger sequencing identified one novel mutation (c.1123_1132delinsTGC). Compared with sequencing, the screening PCR showed 94.1% sensitivity, 100.0% specificity, 100.0% positive predictive value, and 98.5% negative predictive value. Compared with fragment analysis, the screening PCR presented 88.9% sensitivity and 100.0% specificity.
CONCLUSIONS
This screening PCR is a rapid, sensitive, and cost-effective method for the detection of major CALR mutations.
Keyword
MeSH Terms
-
Adult
Aged
Base Sequence
Bone Marrow/metabolism
Calreticulin/chemistry/*genetics/metabolism
DNA Mutational Analysis
Female
Follow-Up Studies
Genotype
Humans
Janus Kinase 2/chemistry/genetics/metabolism
Male
Middle Aged
Mutation
Myeloproliferative Disorders/complications/*diagnosis/genetics
Polymerase Chain Reaction
Thrombocytosis/complications/*diagnosis
Calreticulin
Janus Kinase 2
Figure
Reference
-
1. Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med. 2006; 355:2452–2466. PMID: 17151367.
Article2. Guglielmelli P, Nangalia J, Green AR, Vannucchi AM. CALR mutations in myeloproliferative neoplasms: hidden behind the reticulum. Am J Hematol. 2014; 89:453–456. PMID: 24458922.3. Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013; 369:2391–2405. PMID: 24325359.4. Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013; 369:2379–2390. PMID: 24325356.
Article5. Cabagnols X, Defour J, Ugo V, Ianotto JC, Mossuz P, Mondet J, et al. Differential association of calreticulin type 1 and type 2 mutations with myelofibrosis and essential thrombocytemia: relevance for disease evolution. Leukemia. 2015; 29:249–252. PMID: 25212275.
Article6. Rotunno G, Mannarelli C, Guglielmelli P, Pacilli A, Pancrazzi A, Pieri L, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood. 2014; 123:1552–1555. PMID: 24371211.
Article7. Rumi E, Pietra D, Ferretti V, Klampfl T, Harutyunyan AS, Milosevic JD, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood. 2014; 123:1544–1551. PMID: 24366362.
Article8. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33:159–174. PMID: 843571.
Article9. Tefferi A, Pardanani A. Myeloproliferative Neoplasms: A Contemporary Review. JAMA Oncol. 2015; 1:97–105. PMID: 26182311.10. Qiao C, Sun C, Ouyang Y, Wang JJ, Qian SX, Li JY, et al. Clinical importance of different calreticulin gene mutation types in wild-type JAK2 essential thrombocythemia and myelofibrosis patients. Haematologica. 2014; 99:e182–e184. PMID: 25015940.
Article11. Kim SY, Im K, Park SN, Kwon J, Kim JA, Lee DS. CALR, JAK2, and MPL mutation profiles in patients with four different subtypes of myeloproliferative neoplasms: primary myelofibrosis, essential thrombocythemia, polycythemia vera, and myeloproliferative neoplasm, unclassifiable. Am J Clin Pathol. 2015; 143:635–644. PMID: 25873496.12. Ha JS, Kim YK. Calreticulin exon 9 mutations in myeloproliferative neoplasms. Ann Lab Med. 2015; 35:22–27. PMID: 25553276.
Article13. Jones AV, Ward D, Lyon M, Leung W, Callaway A, Chase A, et al. Evaluation of methods to detect CALR mutations in myeloproliferative neoplasms. Leuk Res. 2015; 39:82–87. PMID: 25499808.
Article14. Chi J, Nicolaou KA, Nicolaidou V, Koumas L, Mitsidou A, Pierides C, et al. Calreticulin gene exon 9 frameshift mutations in patients with thrombocytosis. Leukemia. 2014; 28:1152–1154. PMID: 24365789.
Article15. Lim KH, Chang YC, Gon-Shen Chen C, Lin HC, Wang WT, Chiang YH, et al. Frequent CALR exon 9 alterations in JAK2 V617F-mutated essential thrombocythemia detected by high-resolution melting analysis. Blood Cancer J. 2015; 5:e295. PMID: 25794131.
Article16. Bilbao-Sieyro C, Santana G, Moreno M, Torres L, Santana-Lopez G, Rodriguez-Medina C, et al. High resolution melting analysis: a rapid and accurate method to detect CALR mutations. PLoS One. 2014; 9:e103511. PMID: 25068507.
Article17. Lim KH, Lin HC, Chen CGS, Wang WT, Chang YC, Chiang YH, et al. Rapid and sensitive detection of CALR exon 9 mutations using high-resolution melting analysis. Clin Chim Acta. 2015; 440:133–139. PMID: 25447704.
Article18. Tefferi A, Wassie EA, Guglielmelli P, Gangat N, Belachew AA, Lasho TL, et al. Type 1 versus Type 2 calreticulin mutations in essential thrombocythemia: a collaborative study of 1027 patients. Am J Hematol. 2014; 89:E121–E124. PMID: 24753125.
Article19. Tefferi A, Thiele J, Vannucchi AM, Barbui T. An overview on CALR and CSF3R mutations and a proposal for revision of WHO diagnostic criteria for myeloproliferative neoplasms. Leukemia. 2014; 28:1407–1413. PMID: 24441292.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- KRAS Mutation Test in Korean Patients with Colorectal Carcinomas: A Methodological Comparison between Sanger Sequencing and a Real-Time PCR-Based Assay
- Value of next‑generation sequencing in inherited arrhythmia syndromes
- Philadelphia+ Chronic Myeloid Leukemia with CALR Mutation: A Case Report and Literature Review
- Calreticulin Exon 9 Mutations in Myeloproliferative Neoplasms
- Detection of Rare Mutations in EGFR-ARMS-PCR-Negative Lung Adenocarcinoma by Sanger Sequencing