Ann Lab Med.
2016 May;36(3):275-277. 10.3343/alm.2016.36.3.275.
Septicemia Caused by Neisseria meningitidis With Decreased Ciprofloxacin Susceptibility: The First Case Report in Korea
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, Kyung Hee University School of Medicine, Seoul, Korea. sonjs@korea.com
- 2Department of Laboratory Medicine, Kyung Hee University School of Medicine, Seoul, Korea.
- KMID: 2373542
- DOI: http://doi.org/10.3343/alm.2016.36.3.275
Abstract
- No abstract available.
MeSH Terms
-
Anti-Bacterial Agents/pharmacology
Bacterial Proteins/genetics
Ceftriaxone/pharmacology/therapeutic use
Ciprofloxacin/pharmacology/therapeutic use
DNA, Bacterial/analysis/metabolism
Disk Diffusion Antimicrobial Tests
*Drug Resistance, Bacterial
Female
Humans
Neisseria meningitidis/drug effects/genetics/*isolation & purification
Polymerase Chain Reaction
Sepsis/*diagnosis/drug therapy/microbiology
Transcription Factors/genetics
Young Adult
Anti-Bacterial Agents
Bacterial Proteins
Ceftriaxone
Ciprofloxacin
DNA, Bacterial
Transcription Factors
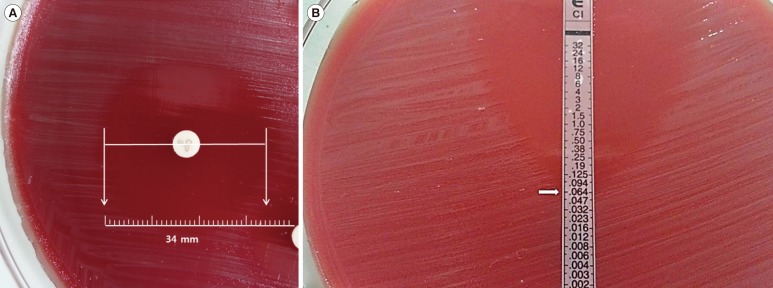
Figure
Reference
-
1. Bae SM, Kang YH. Serological and genetic characterization of meningococcal isolates in Korea. Jpn J Infect Dis. 2008; 61:434–437. PMID: 19050348.2. Clinical and Laboratory Standard Institute. Performance standard for antimicrobial susceptibility testing. Twenty-second Informational supplement, Document M100-S22. Wayne, PA: Clinical and Laboratory Standards Institute;2012.3. Taha MK. Simultaneous approach for nonculture PCR-based identification and serogroup prediction of Neisseria meningitidis. J Clin Microbiol. 2000; 38:855–857. PMID: 10655397.4. The Korean Society of Infectious Diseases. The Korean Society for Chemotherapy. The Korean Neurological Association. The Korean Neurosurgical Society. The Korean Society of Clinical Microbiology. Clinical practice guidelines for the management of bacterial meningitis in adults in Korea. Infect Chemother. 2012; 44:140–163.5. Gaunt PN, Lambert BE. Single dose ciprofloxacin for the eradication of pharyngeal carriage of Neisseria meningitidis. J Antimicrob Chemother. 1988; 21:489–496. PMID: 3132442.
Article6. Mounchetrou Njoya I, Deghmane AE, Taha MK, Isnard H, Parent du Châtelet I. A cluster of meningococcal disease caused by rifampicin-resistant C meningococci in France, April 2012. Euro Surveill. 2012; 17:pii=20254.
Article7. Wu HM, Harcourt BH, Hatcher CP, Wei SC, Novak RT, Wang X, et al. Emergence of ciprofloxacin-resistant Neisseria meningitidis in North America. N Engl J Med. 2009; 360:886–892. PMID: 19246360.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neisseria meningitidis Infections
- Neonatal Sepsis and Meningitis Caused by Neisseria meningitidis Serogroup B: a Case Report
- A Case of Deficiency of the Seventh Component of Complement with Recurrence of Meningococcal Meningitis and Septicemia
- Neisseria subflava Infections: Bacteriological aspects of two cases
- A Case of Meningococcal Sepsis and Meningitis with Complement 7 Deficiency in a Military Trainee