J Gastric Cancer.
2011 Dec;11(4):234-238.
Neuroendocrine Tumor of Unknown Primary Accompanied with Stomach Adenocarcinoma
- Affiliations
-
- 1Department of Surgery, Kyung-Hee University School of Medicine, Seoul, Korea. kyjho@khmc.or.kr
- 2Department of Surgery, Kyung Hee University Hospital at Gangdong, Kyung-Hee University School of Medicine, Seoul, Korea.
Abstract
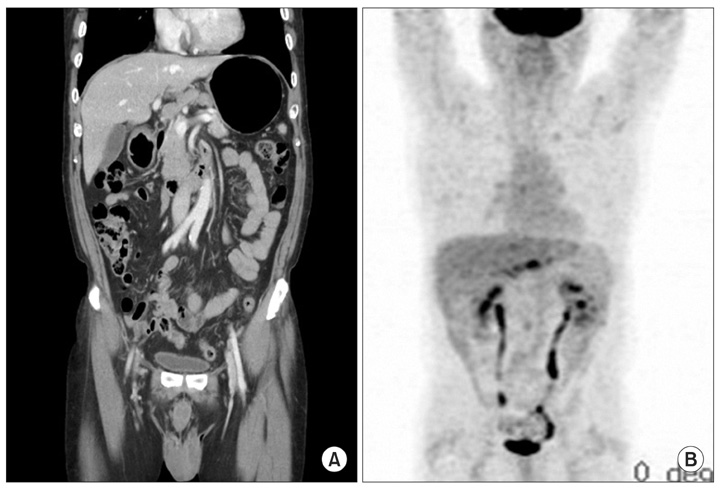
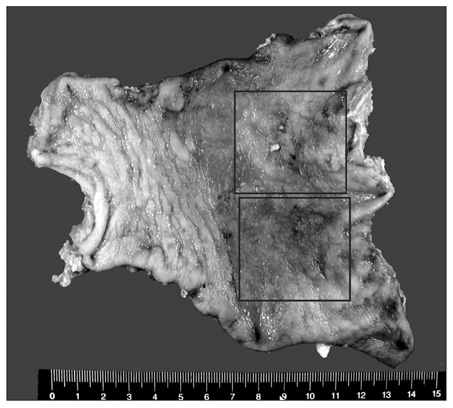
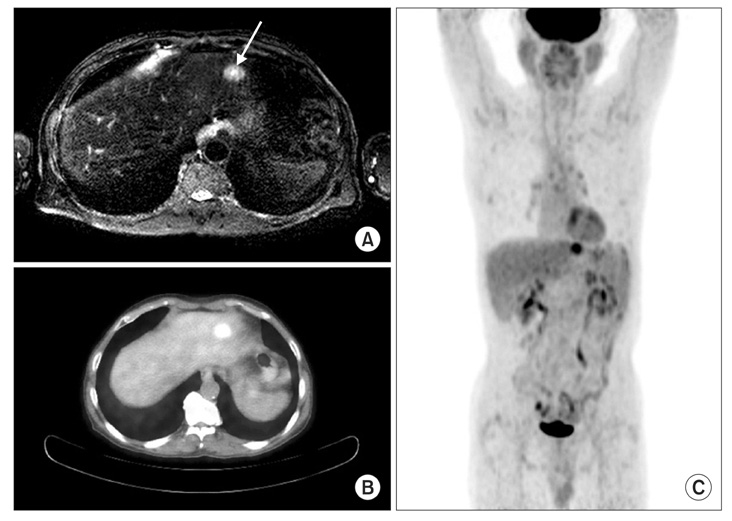
- A 67 year old male at a regular checkup underwent esophagogastroduodenoscopy. On performing esophagogastroduodenoscopy, a lesion about 1.2 cm depressed was noted at the gastric angle. The pathology of the biopsy specimen revealed a well-differentiated adenocarcinoma. On performing an abdominal computed tomography (CT) scan & positron emission tomography-computed tomography (PET-CT) scan, no definite evidence of gastric wall thickening or mass lesion was found. However, lymph node enlargement was found in the left gastric and prepancreatic spaces. This patient underwent laparoscopic assisted distal gastrectomy and D2 lymph node dissection. On final examination, it was found out that the tumor had invaded the mucosal layer. The lymph node was a metastasized large cell neuroendocrine carcinoma with an unknown primary site. The patient refused chemotherapy. He opted to undergo a close follow-up. At the postoperative month 27, he had a focal hypermetabolic lesion in the left lobe of the liver that suggested metastasis on PET-CT scan. He refused to undergo an operation. He underwent a radiofrequency ablation.
MeSH Terms
Figure
Reference
-
1. Fizazi K, Greco FA, Pavlidis N, Pentheroudakis G. ESMO Guidelines Working Group. Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011. 22:Suppl 6. vi64–vi68.
Article2. Stoyianni A, Pentheroudakis G, Pavlidis N. Neuroendocrine carcinoma of unknown primary: a systematic review of the literature and a comparative study with other neuroendocrine tumors. Cancer Treat Rev. 2011. 37:358–365.
Article3. Spigel DR, Hainsworth JD, Greco FA. Neuroendocrine carcinoma of unknown primary site. Semin Oncol. 2009. 36:52–59.
Article4. Tot T. Adenocarcinomas metastatic to the liver: the value of cytokeratins 20 and 7 in the search for unknown primary tumors. Cancer. 1999. 85:171–177.5. Tot T. Cytokeratins 20 and 7 as biomarkers: usefulness in discriminating primary from metastatic adenocarcinoma. Eur J Cancer. 2002. 38:758–763.6. Pavlidis N, Briasoulis E, Hainsworth J, Greco FA. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer. 2003. 39:1990–2005.
Article7. Rindi G, D'Adda T, Froio E, Fellegara G, Bordi C. Prognostic factors in gastrointestinal endocrine tumors. Endocr Pathol. 2007. 18:145–149.
Article8. Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003. 97:934–959.
Article9. Norheim I, Oberg K, Theodorsson-Norheim E, Lindgren PG, Lundqvist G, Magnusson A, et al. Malignant carcinoid tumors. An analysis of 103 patients with regard to tumor localization, hormone production, and survival. Ann Surg. 1987. 206:115–125.
Article10. Oberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol. 2005. 19:753–781.
Article11. Starker LF, Carling T. Molecular genetics of gastroenteropancreatic neuroendocrine tumors. Curr Opin Oncol. 2009. 21:29–33.
Article12. McDermott EW, Guduric B, Brennan MF. Prognostic variables in patients with gastrointestinal carcinoid tumours. Br J Surg. 1994. 81:1007–1009.
Article13. Thompson GB, van Heerden JA, Grant CS, Carney JA, Ilstrup DM. Islet cell carcinomas of the pancreas: a twenty-year experience. Surgery. 1988. 104:1011–1017.14. Zeitels J, Naunheim K, Kaplan EL, Straus F 2nd. Carcinoid tumors: a 37-year experience. Arch Surg. 1982. 117:732–737.15. Wang SC, Parekh JR, Zuraek MB, Venook AP, Bergsland EK, Warren RS, et al. Identification of unknown primary tumors in patients with neuroendocrine liver metastases. Arch Surg. 2010. 145:276–280.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Metastatic Large Cell Neuroendocrine Carcinoma Combined with Gastric Adenocarcinoma
- CT Gastrography Findings of a Gastric Collision Tumor that Consisted of an Adenocarcinoma and Neuroendocrine Tumor: A Case Report
- Composite Neuroendocrine Carcinoma with Adenocarcinoma of the Stomach Misdiagnosed as a Giant Submucosal Tumor
- Gastric Collision Tumor Consisting of Mucinous Carcinoma and Large Cell Neuroendocrine Carcinoma: A Case Report
- A Case of a Gastric Composite Tumor with an Adenocarcinoma and a Large Cell Neuroendocrine Carcinoma