J Bone Metab.
2017 Feb;24(1):23-30. 10.11005/jbm.2017.24.1.23.
Effects of Escherichia Coli-derived Recombinant Human Bone Morphogenetic Protein-2 Loaded Porous Hydroxyaptite-based Ceramics on Calvarial Defect in Rabbits
- Affiliations
-
- 1Department of Periodontology, School of Dentistry, Kyungpook National University, Daegu, Korea. periokyg@knu.ac.kr
- 2Department of Biochemistry, School of Dentistry, Kyungpook National University, Daegu, Korea.
- 3Institute for Hard Tissue and Bone Regeneration (IHBR), Kyungpook National University, Daegu, Korea.
- 4Biomedical Engineering and Radiology, School of Medicine, Catholic University of Daegu, School of Medicine, Daegu, Korea.
- 5Industrial Technology Convergence Center, Pohang Accelerator Laboratory, Pohang University of Science and Technology (POSTECH), Pohang, Korea.
- KMID: 2371829
- DOI: http://doi.org/10.11005/jbm.2017.24.1.23
Abstract
- BACKGROUND
Recombinant human bone morphogenetic proteins (rhBMPs) have been widely used in regenerative therapies to promote bone formation. The production of rhBMPs using bacterial systems such as Escherichia coli (E. coli) is estimated to facilitate clinical applications by lowering the cost without compromising biological activity. In clinical practice, rhBMP-2 and osteoconductive carriers (e.g., hydroxyapatite [HA] and bovine bone xenograft) are used together. This study examined the effect of E. coli-derived rhBMP-2 combined with porous HA-based ceramics on calvarial defect in rabbits.
METHODS
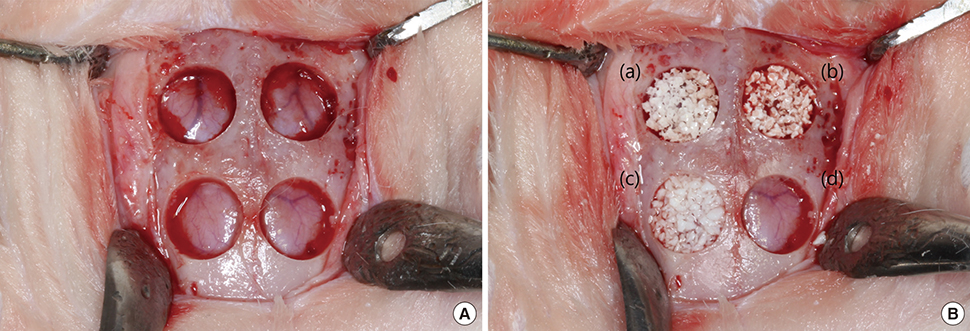
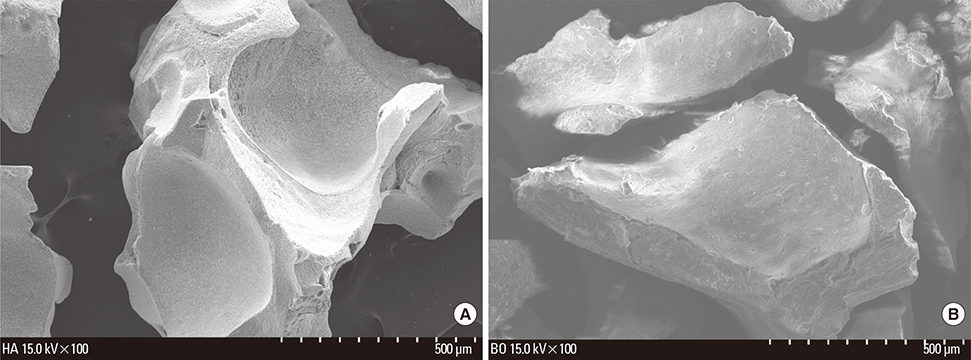
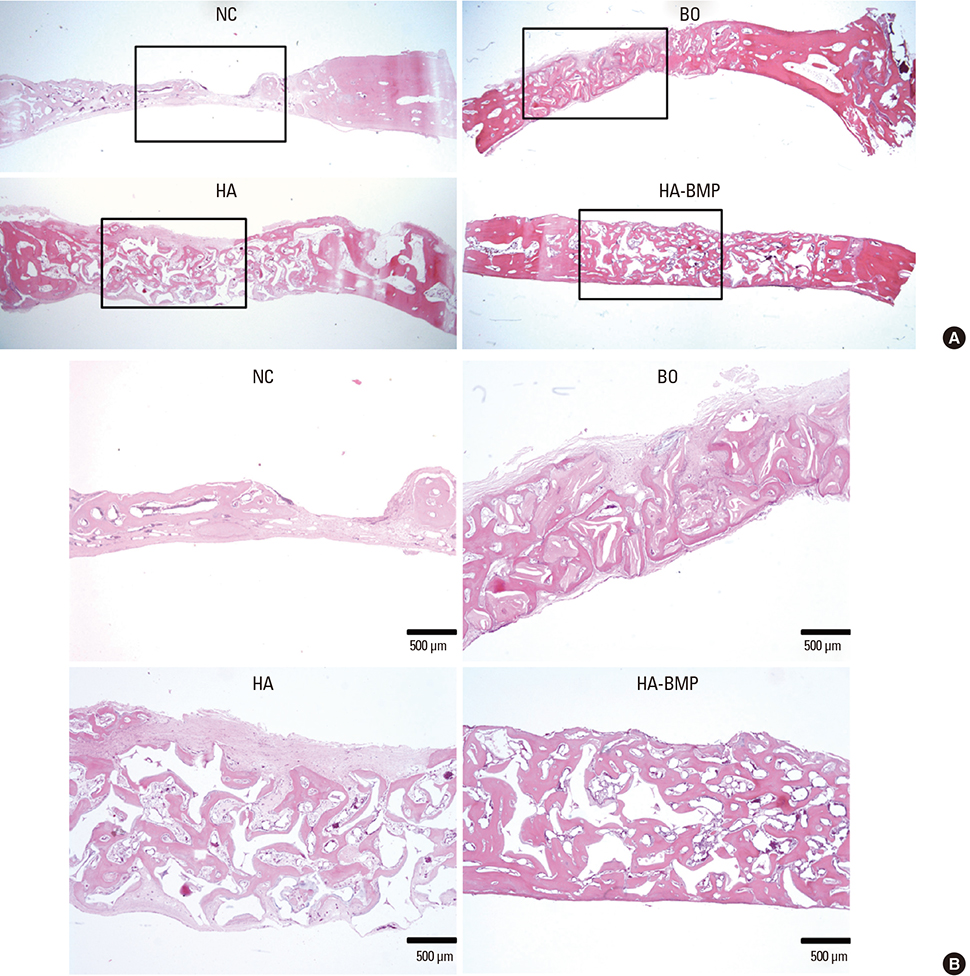
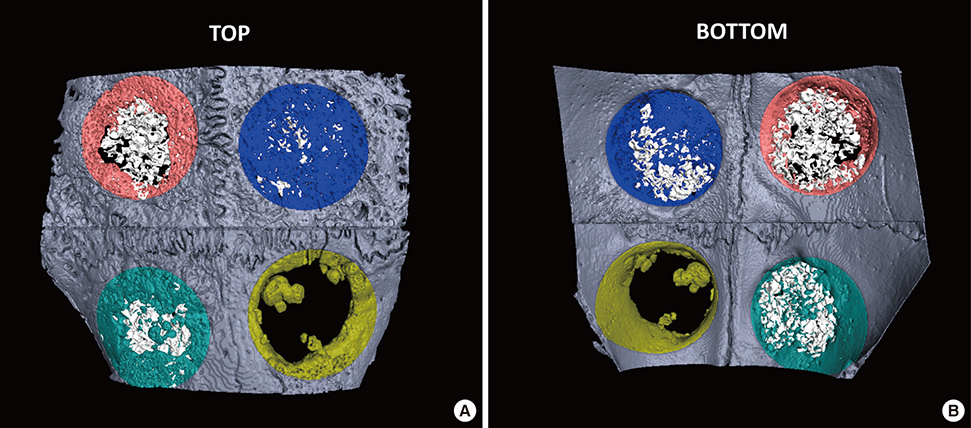
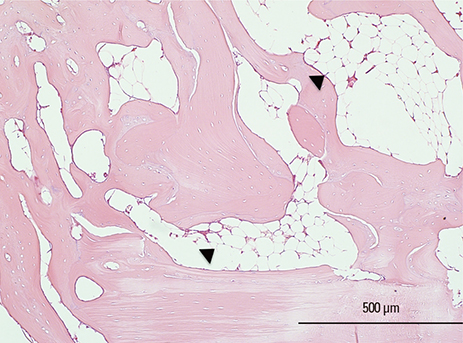
Six adult male New Zealand white rabbits were used in this study. The experimental groups were divided into the following 4 groups: untreated (NC), bovine bone graft (BO), porous HA (HA) and porous HA with rhBMP-2 (HA-BMP). Four transosseous defects of 8 mm in diameter were prepared using stainless steel trephine bur in the frontal and parietal bones. Histological and histomorphometric analyses at 4 weeks after surgery revealed significant new bone formation by porous HA alone.
RESULTS
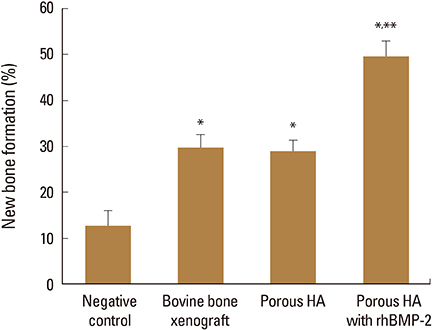
HA-BMP showed significantly higher degree of bone formation compared with BO and HA group (P<0.05). The average new bone formation % (new bone area per total defect area) of NC, BO, HA, and HA-BMP at 4-week after surgery were 12.65±5.89%, 29.63±6.99%, 28.86±6.17% and 49.56±8.23%, respectively. However, there was no statistical difference in the bone formation between HA and BO groups.
CONCLUSIONS
HA-BMP promoted more bone formation than NC, BO and HA alone. Thus, using E. coli-derived rhBMP-2 combined with porous HA-based ceramics can promote new bone formation.
MeSH Terms
Figure
Reference
-
1. Urist MR. Bone: formation by autoinduction. Science. 1965; 150:893–899.
Article2. Reddi AH, Cunningham NS. Initiation and promotion of bone differentiation by bone morphogenetic proteins. J Bone Miner Res. 1993; 8:Suppl 2. S499–S502.
Article3. Inoda H, Yamamoto G, Hattori T. Histological investigation of osteoinductive properties of rh-BMP2 in a rat calvarial bone defect model. J Craniomaxillofac Surg. 2004; 32:365–369.
Article4. Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996; 10:1580–1594.
Article5. Sigurdsson TJ, Nygaard L, Tatakis DN, et al. Periodontal repair in dogs: evaluation of rhBMP-2 carriers. Int J Periodontics Restorative Dent. 1996; 16:524–537.6. Urist MR, Huo YK, Brownell AG, et al. Purification of bovine bone morphogenetic protein by hydroxyapatite chromatography. Proc Natl Acad Sci U S A. 1984; 81:371–375.
Article7. Israel DI, Nove J, Kerns KM, et al. Expression and characterization of bone morphogenetic protein-2 in Chinese hamster ovary cells. Growth Factors. 1992; 7:139–150.
Article8. Lee JH, Jang SJ, Koo TY, et al. Expression, purification and osteogenic bioactivity of recombinant human BMP-2 derived by Escherichia coli. Tissue Eng Regen Med. 2011; 8:8–15.9. Vallejo LF, Brokelmann M, Marten S, et al. Renaturation and purification of bone morphogenetic protein-2 produced as inclusion bodies in high-cell-density cultures of recombinant Escherichia coli. J Biotechnol. 2002; 94:185–194.
Article10. Lee J, Lee EN, Yoon J, et al. Comparative study of Chinese hamster ovary cell versus Escherichia coli-derived bone morphogenetic protein-2 using the critical-size supraalveolar peri-implant defect model. J Periodontol. 2013; 84:415–422.
Article11. Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003; 55:1613–1629.
Article12. Daculsi G, LeGeros RZ, Nery E, et al. Transformation of biphasic calcium phosphate ceramics in vivo: ultrastructural and physicochemical characterization. J Biomed Mater Res. 1989; 23:883–894.
Article13. Lee JH, Hwang CJ, Song BW, et al. A prospective consecutive study of instrumented posterolateral lumbar fusion using synthetic hydroxyapatite (Bongros-HA) as a bone graft extender. J Biomed Mater Res A. 2009; 90:804–810.
Article14. Bignon A, Chouteau J, Chevalier J, et al. Effect of micro- and macroporosity of bone substitutes on their mechanical properties and cellular response. J Mater Sci Mater Med. 2003; 14:1089–1097.
Article15. Tsuruga E, Takita H, Itoh H, et al. Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. J Biochem. 1997; 121:317–324.
Article16. Ripamonti U, Ma SS, van den Heever B, et al. Osteogenin, a bone morphogenetic protein, adsorbed on porous hydroxyapatite substrata, induces rapid bone differentiation in calvarial defects of adult primates. Plast Reconstr Surg. 1992; 90:382–393.
Article17. Langer R, Vacanti JP. Tissue engineering. Science. 1993; 260:920–926.
Article18. Murphy CM, Haugh MG, O'Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 2010; 31:461–466.
Article19. Liebschner MA. Biomechanical considerations of animal models used in tissue engineering of bone. Biomaterials. 2004; 25:1697–1714.
Article20. Jones JR, Hench LL. Factors affecting the structure and properties of bioactive foam scaffolds for tissue engineering. J Biomed Mater Res B Appl Biomater. 2004; 68:36–44.
Article21. Vallet-Regí M. Current trends on porous inorganic materials for biomedical applications. Chem Eng J. 2008; 137:1–3.
Article22. Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005; 26:5474–5491.
Article23. Rohanizadeh R, Chung K. Hydroxyapatite as a carrier for bone morphogenetic protein. J Oral Implantol. 2011; 37:659–672.
Article24. Sciadini MF, Johnson KD. Evaluation of recombinant human bone morphogenetic protein-2 as a bone-graft substitute in a canine segmental defect model. J Orthop Res. 2000; 18:289–302.
Article25. Park JC, Kim JC, Kim BK, et al. Dose- and time-dependent effects of recombinant human bone morphogenetic protein-2 on the osteogenic and adipogenic potentials of alveolar bone-derived stromal cells. J Periodontal Res. 2012; 47:645–654.
Article26. Yoshida K, Bessho K, Fujimura K, et al. Enhancement by recombinant human bone morphogenetic protein-2 of bone formation by means of porous hydroxyapatite in mandibular bone defects. J Dent Res. 1999; 78:1505–1510.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Block Bone Substitutes Loaded with Escherichia Coli-Produced Recombinant Human Bone Morphogenetic Protein-2 on Space Maintenance and Bone Formation in Rat Calvarial Onlay Model
- Bone Healing in Ovariectomized-rabbit Calvarial Defect with Tricalcium Phosphate Coated with Recombinant Human Bone Morphogenetic Protein-2 Genetically Engineered in Escherichia coli
- Effect of rhBMP-2 produced by Escherichia coli expression system on bone formation in rat calvarial defects
- Paracrine effect of the bone morphogeneticprotein-2 at the experimental site on healing of the adjacent control site: a study in the rabbit calvarial defect model
- Acute Intravenous Injection Toxicity Study of Escherichia coli-Derived Recombinant Human Bone Morphogenetic Protein-2 in Rat