Korean J Physiol Pharmacol.
2017 Jan;21(1):37-44. 10.4196/kjpp.2017.21.1.37.
Regulation of vascular smooth muscle phenotype by cross-regulation of krüppel-like factors
- Affiliations
-
- 1Gene and Cell Therapy for Vessel-Associated Disease, Medical Research Institute, Department of Pharmacology, Pusan National University School of Medicine, Yangsan 50612, Korea. sunsik@pusan.ac.kr
- 2Department of Physics, Dong-A University, Busan 49315, Korea.
- 3Department of Anatomy, Pusan National University School of Korean Medicine, Yangsan 50612, Korea.
- KMID: 2371066
- DOI: http://doi.org/10.4196/kjpp.2017.21.1.37
Abstract
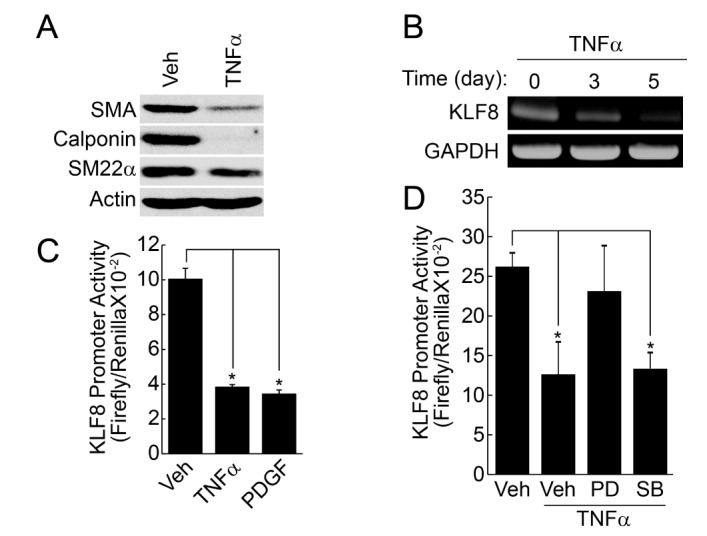
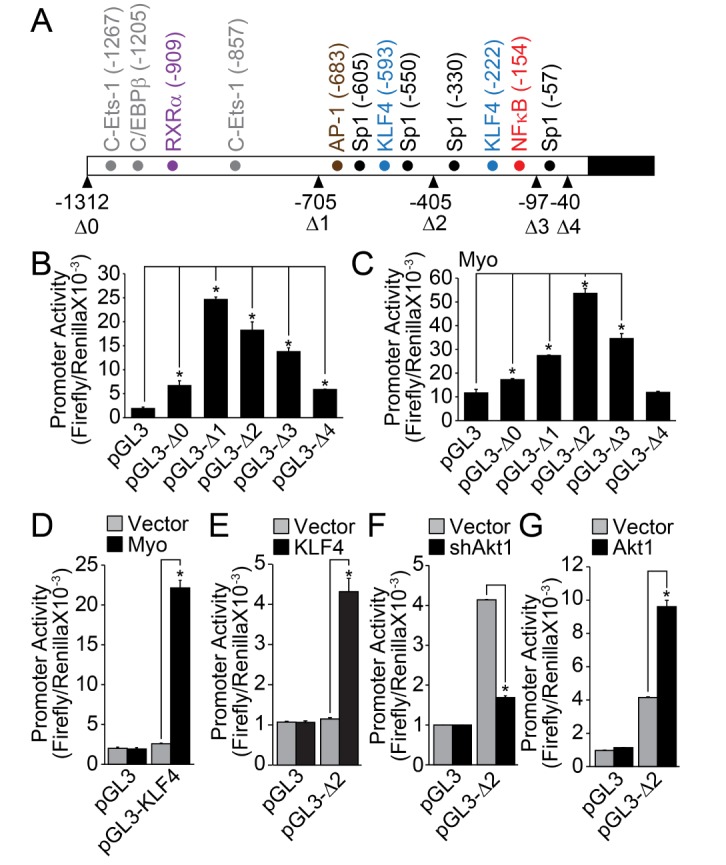
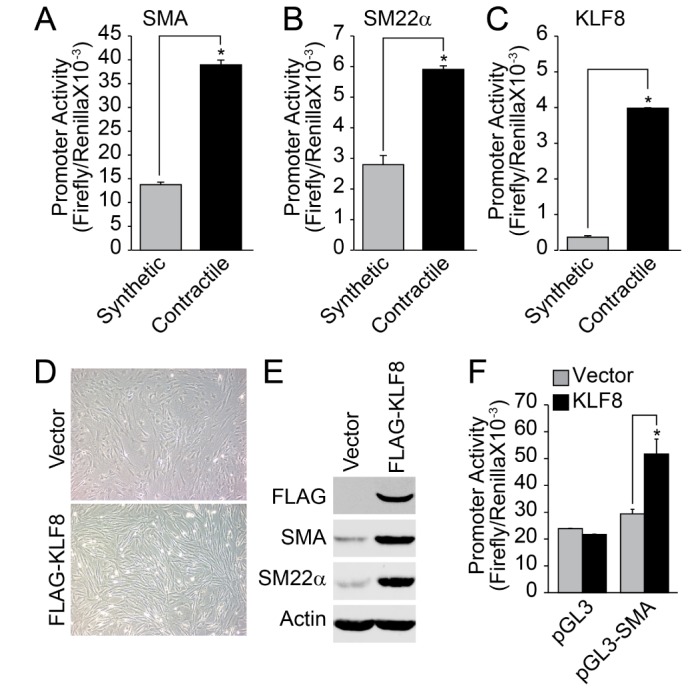
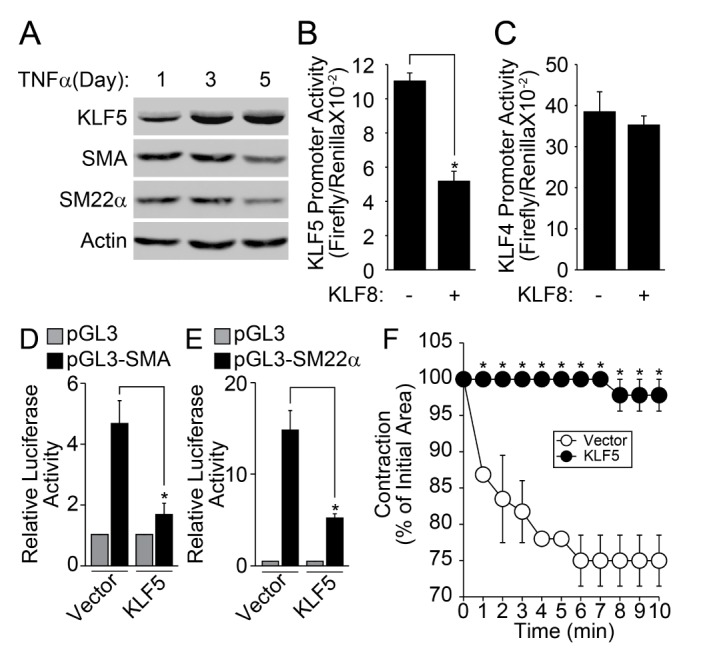
- Regulation of vascular smooth muscle cell (VSMC) phenotype plays an essential role in many cardiovascular diseases. In the present study, we provide evidence that krüppel-like factor 8 (KLF8) is essential for tumor necrosis factor α (TNFα)-induced phenotypic conversion of VSMC obtained from thoracic aorta from 4-week-old SD rats. Stimulation of the contractile phenotype of VSMCs with TNFα significantly reduced the VSMC marker gene expression and KLF8. The gene expression of KLF8 was blocked by TNFα stimulation in an ERK-dependent manner. The promoter region of KLF8 contained putative Sp1, KLF4, and NFκB binding sites. Myocardin significantly enhanced the promoter activity of KLF4 and KLF8. The ectopic expression of KLF4 strongly enhanced the promoter activity of KLF8. Moreover, silencing of Akt1 significantly attenuated the promoter activity of KLF8; conversely, the overexpression of Akt1 significantly enhanced the promoter activity of KLF8. The promoter activity of SMA, SM22α, and KLF8 was significantly elevated in the contractile phenotype of VSMCs. The ectopic expression of KLF8 markedly enhanced the expression of SMA and SM22α concomitant with morphological changes. The overexpression of KLF8 stimulated the promoter activity of SMA. Stimulation of VSMCs with TNFα enhanced the expression of KLF5, and the promoter activity of KLF5 was markedly suppressed by KLF8 ectopic expression. Finally, the overexpression of KLF5 suppressed the promoter activity of SMA and SM22α, thereby reduced the contractility in response to the stimulation of angiotensin II. These results suggest that cross-regulation of KLF family of transcription factors plays an essential role in the VSMC phenotype.
MeSH Terms
-
Angiotensin II
Angiotensins
Animals
Aorta, Thoracic
Binding Sites
Cardiovascular Diseases
Ectopic Gene Expression
Gene Expression
Humans
Muscle, Smooth, Vascular*
Phenotype*
Promoter Regions, Genetic
Rats
Transcription Factors
Tumor Necrosis Factor-alpha
Angiotensin II
Angiotensins
Transcription Factors
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995; 75:487–517. PMID: 7624392.
Article2. Wynne BM, Chiao CW, Webb RC. Vascular smooth muscle cell signaling mechanisms for contraction to angiotensin ii and endothelin-1. J Am Soc Hypertens. 2009; 3:84–95. PMID: 20161229.
Article3. Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004; 84:767–801. PMID: 15269336.
Article4. McConnell BB, Yang VW. Mammalian Krüppel-like factors in health and diseases. Physiol Rev. 2010; 90:1337–1381. PMID: 20959618.
Article5. Haldar SM, Ibrahim OA, Jain MK. Kruppel-like factors (KLFs) in muscle biology. J Mol Cell Cardiol. 2007; 43:1–10. PMID: 17531262.
Article6. Evans PM, Zhang W, Chen X, Yang J, Bhakat KK, Liu C. Kruppel-like factor 4 is acetylated by p300 and regulates gene transcription via modulation of histone acetylation. J Biol Chem. 2007; 282:33994–34002. PMID: 17908689.7. Miyamoto S, Suzuki T, Muto S, Aizawa K, Kimura A, Mizuno Y, Nagino T, Imai Y, Adachi N, Horikoshi M, Nagai R. Positive and negative regulation of the cardiovascular transcription factor KLF5 by p300 and the oncogenic regulator SET through interaction and acetylation on the DNA-binding domain. Mol Cell Biol. 2003; 23:8528–8541. PMID: 14612398.
Article8. Matsumura T, Suzuki T, Aizawa K, Munemasa Y, Muto S, Horikoshi M, Nagai R. The deacetylase HDAC1 negatively regulates the cardiovascular transcription factor Krüppel-like factor 5 through direct interaction. J Biol Chem. 2005; 280:12123–12129. PMID: 15668237.
Article9. Meng F, Han M, Zheng B, Wang C, Zhang R, Zhang XH, Wen JK. All-trans retinoic acid increases KLF4 acetylation by inducing HDAC2 phosphorylation and its dissociation from KLF4 in vascular smooth muscle cells. Biochem Biophys Res Commun. 2009; 387:13–18. PMID: 19486889.
Article10. He M, Han M, Zheng B, Shu YN, Wen JK. Angiotensin II stimulates KLF5 phosphorylation and its interaction with c-Jun leading to suppression of p21 expression in vascular smooth muscle cells. J Biochem. 2009; 146:683–691. PMID: 19628677.
Article11. Oishi Y, Manabe I, Tobe K, Ohsugi M, Kubota T, Fujiu K, Maemura K, Kubota N, Kadowaki T, Nagai R. SUMOylation of Krüppel-like transcription factor 5 acts as a molecular switch in transcriptional programs of lipid metabolism involving PPAR-delta. Nat Med. 2008; 14:656–666. PMID: 18500350.12. Chen ZY, Wang X, Zhou Y, Offner G, Tseng CC. Destabilization of Krüppel-like factor 4 protein in response to serum stimulation involves the ubiquitin-proteasome pathway. Cancer Res. 2005; 65:10394–10400. PMID: 16288030.
Article13. Chen C, Sun X, Ran Q, Wilkinson KD, Murphy TJ, Simons JW, Dong JT. Ubiquitin-proteasome degradation of KLF5 transcription factor in cancer and untransformed epithelial cells. Oncogene. 2005; 24:3319–3327. PMID: 15735697.
Article14. Du JX, Yun CC, Bialkowska A, Yang VW. Protein inhibitor of activated STAT1 interacts with and up-regulates activities of the pro-proliferative transcription factor Krüppel-like factor 5. J Biol Chem. 2007; 282:4782–4793. PMID: 17178721.
Article15. Kawai-Kowase K, Kumar MS, Hoofnagle MH, Yoshida T, Owens GK. PIAS1 activates the expression of smooth muscle cell differentiation marker genes by interacting with serum response factor and class I basic helix-loop-helix proteins. Mol Cell Biol. 2005; 25:8009–8023. PMID: 16135793.
Article16. Du JX, Bialkowska AB, McConnell BB, Yang VW. SUMOylation regulates nuclear localization of Krüppel-like factor 5. J Biol Chem. 2008; 283:31991–32002. PMID: 18782761.
Article17. Beamish JA, He P, Kottke-Marchant K, Marchant RE. Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng Part B Rev. 2010; 16:467–491. PMID: 20334504.
Article18. Kober F, Canault M, Peiretti F, Mueller C, Kopp F, Alessi MC, Cozzone PJ, Nalbone G, Bernard M. MRI follow-up of TNF-dependent differential progression of atherosclerotic wall-thickening in mouse aortic arch from early to advanced stages. Atherosclerosis. 2007; 195:e93–e99. PMID: 17662986.
Article19. Ohta H, Wada H, Niwa T, Kirii H, Iwamoto N, Fujii H, Saito K, Sekikawa K, Seishima M. Disruption of tumor necrosis factor-alpha gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis. 2005; 180:11–17. PMID: 15823270.20. Yun SJ, Ha JM, Kim EK, Kim YW, Jin SY, Lee DH, Song SH, Kim CD, Shin HK, Bae SS. Akt1 isoform modulates phenotypic conversion of vascular smooth muscle cells. Biochim Biophys Acta. 2014; 1842:2184–2192. PMID: 25201081.
Article21. Ha JM, Yun SJ, Kim YW, Jin SY, Lee HS, Song SH, Shin HK, Bae SS. Platelet-derived growth factor regulates vascular smooth muscle phenotype via mammalian target of rapamycin complex 1. Biochem Biophys Res Commun. 2015; 464:57–62. PMID: 26032503.
Article22. Kim SH, Yun SJ, Kim YH, Ha JM, Jin SY, Lee HS, Kim SJ, Shin HK, Chung SW, Bae SS. Essential role of krüppel-like factor 5 during tumor necrosis factor α-induced phenotypic conversion of vascular smooth muscle cells. Biochem Biophys Res Commun. 2015; 463:1323–1327. PMID: 26102029.
Article23. Hayashi K, Shibata K, Morita T, Iwasaki K, Watanabe M, Sobue K. Insulin receptor substrate-1/SHP-2 interaction, a phenotype-dependent switching machinery of insulin-like growth factor-I signaling in vascular smooth muscle cells. J Biol Chem. 2004; 279:40807–40818. PMID: 15272025.
Article24. Wang CC, Gurevich I, Draznin B. Insulin affects vascular smooth muscle cell phenotype and migration via distinct signaling pathways. Diabetes. 2003; 52:2562–25629. PMID: 14514641.
Article25. van Vliet J, Turner J, Crossley M. Human Krüppel-like factor 8: a CACCC-box binding protein that associates with CtBP and represses transcription. Nucleic Acids Res. 2000; 28:1955–1962. PMID: 10756197.26. Gao D, Niu X, Ning N, Hao G. Regulation of angiotensin II-Induced Krüppel-like factor 5 expression in vascular smooth muscle cells. Biol Pharm Bull. 2006; 29:2004–2008. PMID: 17015941.27. Garvey SM, Sinden DS, Schoppee Bortz PD, Wamhoff BR. Cyclosporine up-regulates Krüppel-like factor-4 (KLF4) in vascular smooth muscle cells and drives phenotypic modulation in vivo. J Pharmacol Exp Ther. 2010; 333:34–42. PMID: 20089806.
Article28. Liu Y, Sinha S, McDonald OG, Shang Y, Hoofnagle MH, Owens GK. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J Biol Chem. 2005; 280:9719–9727. PMID: 15623517.
Article29. Nagai R, Suzuki T, Aizawa K, Shindo T, Manabe I. Significance of the transcription factor KLF5 in cardiovascular remodeling. J Thromb Haemost. 2005; 3:1569–1576. PMID: 16102021.
Article30. Yoshida T, Kaestner KH, Owens GK. Conditional deletion of Krüppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ Res. 2008; 102:1548–1557. PMID: 18483411.31. Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001; 105:851–862. PMID: 11439182.
Article32. Yoshida T, Sinha S, Dandré F, Wamhoff BR, Hoofnagle MH, Kremer BE, Wang DZ, Olson EN, Owens GK. Myocardin is a key regulator of CArG-dependent transcription of multiple smooth muscle marker genes. Circ Res. 2003; 92:856–864. PMID: 12663482.
Article33. Li HX, Han M, Bernier M, Zheng B, Sun SG, Su M, Zhang R, Fu JR, Wen JK. Krüppel-like factor 4 promotes differentiation by transforming growth factor-beta receptor-mediated Smad and p38 MAPK signaling in vascular smooth muscle cells. J Biol Chem. 2010; 285:17846–17856. PMID: 20375011.34. Agarwal A, Das K, Lerner N, Sathe S, Cicek M, Casey G, Sizemore N. The AKT/I kappa B kinase pathway promotes angiogenic/metastatic gene expression in colorectal cancer by activating nuclear factor-kappa B and beta-catenin. Oncogene. 2005; 24:1021–1031. PMID: 15592509.35. Mansell A, Khelef N, Cossart P, O'Neill LA. Internalin B activates nuclear factor-kappa B via Ras, phosphoinositide 3-kinase, and Akt. J Biol Chem. 2001; 276:43597–43603. PMID: 11571285.36. Yang CH, Murti A, Pfeffer SR, Kim JG, Donner DB, Pfeffer LM. Interferon alpha /beta promotes cell survival by activating nuclear factor kappa B through phosphatidylinositol 3-kinase and Akt. J Biol Chem. 2001; 276:13756–13761. PMID: 11278812.37. Wang X, Urvalek AM, Liu J, Zhao J. Activation of KLF8 transcription by focal adhesion kinase in human ovarian epithelial and cancer cells. J Biol Chem. 2008; 283:13934–13942. PMID: 18353772.
Article38. Suzuki T, Sawaki D, Aizawa K, Munemasa Y, Matsumura T, Ishida J, Nagai R. Kruppel-like factor 5 shows proliferation-specific roles in vascular remodeling, direct stimulation of cell growth, and inhibition of apoptosis. J Biol Chem. 2009; 284:9549–9557. PMID: 19189969.39. Bafford R, Sui XX, Wang G, Conte M. Angiotensin II and tumor necrosis factor-alpha upregulate survivin and Kruppel-like factor 5 in smooth muscle cells: Potential relevance to vein graft hyperplasia. Surgery. 2006; 140:289–296. PMID: 16904982.
Article40. Hoshino Y, Kurabayashi M, Kanda T, Hasegawa A, Sakamoto H, Okamoto E, Kowase K, Watanabe N, Manabe I, Suzuki T, Nakano A, Takase S, Wilcox JN, Nagai R. Regulated expression of the BTEB2 transcription factor in vascular smooth muscle cells: analysis of developmental and pathological expression profiles shows implications as a predictive factor for restenosis. Circulation. 2000; 102:2528–2554. PMID: 11076828.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tyrosine phosphorylation of paxillin may be involved in vascular smooth muscle contraction
- Regulation of Smooth Muscle Excitability
- Regulation of Smooth Muscle Excitability
- The Contribution of Resident Vascular Stem Cells to Arterial Pathology
- Lactate promotes vascular smooth muscle cell switch to a synthetic phenotype by inhibiting miR-23b expression