Korean J Physiol Pharmacol.
2017 Mar;21(2):259-265. 10.4196/kjpp.2017.21.2.259.
Chronic Ca²⺠influx through voltage-dependent Ca²⺠channels enhance delayed rectifier K⺠currents via activating Src family tyrosine kinase in rat hippocampal neurons
- Affiliations
-
- 1Department of Physiology, School of Medicine, Jeju National University, Jeju 63243, Korea. jungsc@jejunu.ac.kr
- 2Institute of Medical Science, Jeju National University, Jeju 63243, Korea.
- 3Department of Physiology, College of Medicine, Konyang University, Daejeon 35365, Korea.
- KMID: 2371045
- DOI: http://doi.org/10.4196/kjpp.2017.21.2.259
Abstract
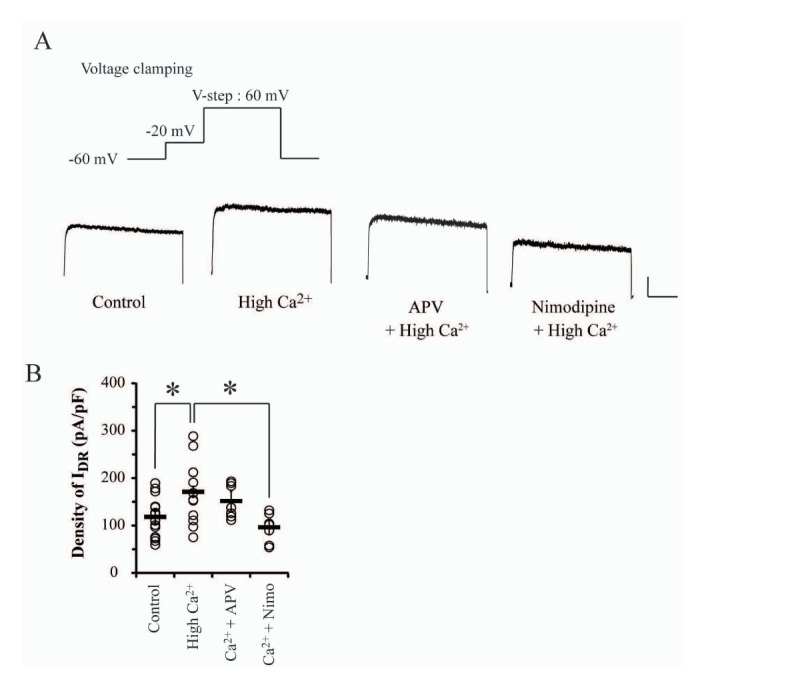
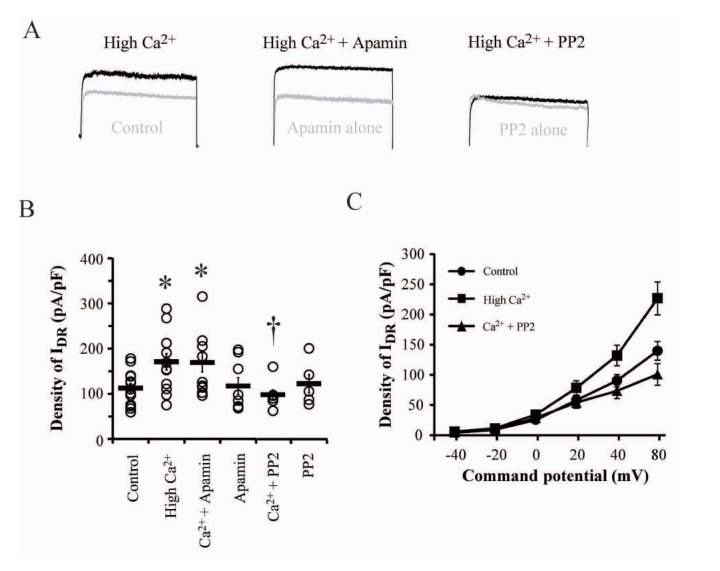
- Excessive influx and the subsequent rapid cytosolic elevation of Ca²âº in neurons is the major cause to induce hyperexcitability and irreversible cell damage although it is an essential ion for cellular signalings. Therefore, most neurons exhibit several cellular mechanisms to homeostatically regulate cytosolic Ca²âº level in normal as well as pathological conditions. Delayed rectifier K⺠channels (I(DR) channels) play a role to suppress membrane excitability by inducing K⺠outflow in various conditions, indicating their potential role in preventing pathogenic conditions and cell damage under Ca²âº-mediated excitotoxic conditions. In the present study, we electrophysiologically evaluated the response of IDR channels to hyperexcitable conditions induced by high Ca²âº pretreatment (3.6 mM, for 24 hours) in cultured hippocampal neurons. In results, high Ca²âº-treatment significantly increased the amplitude of IDR without changes of gating kinetics. Nimodipine but not APV blocked Ca²âº-induced IDR enhancement, confirming that the change of I(DR) might be targeted by Ca²âº influx through voltage-dependent Ca²âº channels (VDCCs) rather than NMDA receptors (NMDARs). The VDCC-mediated I(DR) enhancement was not affected by either Ca²âº-induced Ca²âº release (CICR) or small conductance Ca²âº-activated K⺠channels (SK channels). Furthermore, PP2 but not H89 completely abolished I(DR) enhancement under high Ca²âº condition, indicating that the activation of Src family tyrosine kinases (SFKs) is required for Ca²âº-mediated I(DR) enhancement. Thus, SFKs may be sensitive to excessive Ca²âº influx through VDCCs and enhance I(DR) to activate a neuroprotective mechanism against Ca²âº-mediated hyperexcitability in neurons.
Keyword
MeSH Terms
Figure
Reference
-
1. Misonou H, Mohapatra DP, Trimmer JS. Kv2.1: a voltage-gated K+ channel critical to dynamic control of neuronal excitability. Neurotoxicology. 2005; 26:743–752. PMID: 15950285.2. Oliver D, Lien CC, Soom M, Baukrowitz T, Jonas P, Fakler B. Functional conversion between A-type and delayed rectifier K+ channels by membrane lipids. Science. 2004; 304:265–270. PMID: 15031437.3. Du J, Haak LL, Phillips-Tansey E, Russell JT, McBain CJ. Frequency-dependent regulation of rat hippocampal somato-dendritic excitability by the K+ channel subunit Kv2.1. J Physiol. 2000; 522:19–31. PMID: 10618149.4. Malin SA, Nerbonne JM. Delayed rectifier K+ currents, IK, are encoded by Kv2 alpha-subunits and regulate tonic firing in mammalian sympathetic neurons. J Neurosci. 2002; 22:10094–10105. PMID: 12451110.5. Pal S, Hartnett KA, Nerbonne JM, Levitan ES, Aizenman E. Mediation of neuronal apoptosis by Kv2.1-encoded potassium channels. J Neurosci. 2003; 23:4798–4802. PMID: 12832499.
Article6. Lien CC, Jonas P. Kv3 potassium conductance is necessary and kinetically optimized for high-frequency action potential generation in hippocampal interneurons. J Neurosci. 2003; 23:2058–2068. PMID: 12657664.
Article7. Surmeier DJ, Foehring R. A mechanism for homeostatic plasticity. Nat Neurosci. 2004; 7:691–692. PMID: 15220926.
Article8. Mohapatra DP, Trimmer JS. The Kv2.1 C terminus can autonomously transfer Kv2.1-like phosphorylation-dependent localization, voltage-dependent gating, and muscarinic modulation to diverse Kv channels. J Neurosci. 2006; 26:685–695. PMID: 16407566.
Article9. Jonas EA, Kaczmarek LK. Regulation of potassium channels by protein kinases. Curr Opin Neurobiol. 1996; 6:318–323. PMID: 8794088.
Article10. Murakoshi H, Shi G, Scannevin RH, Trimmer JS. Phosphorylation of the Kv2.1 K+ channel alters voltage-dependent activation. Mol Pharmacol. 1997; 52:821–828. PMID: 9351973.11. Misonou H, Mohapatra DP, Menegola M, Trimmer JS. Calcium- and metabolic state-dependent modulation of the voltage-dependent Kv2.1 channel regulates neuronal excitability in response to ischemia. J Neurosci. 2005; 25:11184–11193. PMID: 16319318.
Article12. Misonou H, Mohapatra DP, Park EW, Leung V, Zhen D, Misonou K, Anderson AE, Trimmer JS. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat Neurosci. 2004; 7:711–718. PMID: 15195093.
Article13. Park KS, Mohapatra DP, Misonou H, Trimmer JS. Graded regulation of the Kv2.1 potassium channel by variable phosphorylation. Science. 2006; 313:976–979. PMID: 16917065.
Article14. Kang MS, Yang YS, Kim SH, Park JM, Eun SY, Jung SC. The down-regulation of somatic a-type K+ channels requires the activation of synaptic nmda receptors in young hippocampal neurons of rats. Korean J Physiol Pharmacol. 2014; 18:135–141. PMID: 24757375.15. Kim J, Jung SC, Clemens AM, Petralia RS, Hoffman DA. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron. 2007; 54:933–947. PMID: 17582333.16. Jung SC, Kim J, Hoffman DA. Rapid, bidirectional remodeling of synaptic NMDA receptor subunit composition by A-type K+ channel activity in hippocampal CA1 pyramidal neurons. Neuron. 2008; 60:657–671. PMID: 19038222.17. Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda). 2006; 21:69–78. PMID: 16443824.
Article18. Hammond RS, Lin L, Sidorov MS, Wikenheiser AM, Hoffman DA. Protein kinase a mediates activity-dependent Kv4.2 channel trafficking. J Neurosci. 2008; 28:7513–7519. PMID: 18650329.
Article19. Kim J, Wei DS, Hoffman DA. Kv4 potassium channel subunits control action potential repolarization and frequency-dependent broadening in rat hippocampal CA1 pyramidal neurones. J Physiol. 2005; 569:41–57. PMID: 16141270.
Article20. Bekkers JM. Distribution and activation of voltage-gated potassium channels in cell-attached and outside-out patches from large layer 5 cortical pyramidal neurons of the rat. J Physiol. 2000; 525:611–620. PMID: 10856116.
Article21. Guan D, Tkatch T, Surmeier DJ, Armstrong WE, Foehring RC. Kv2 subunits underlie slowly inactivating potassium current in rat neocortical pyramidal neurons. J Physiol. 2007; 581:941–960. PMID: 17379638.
Article22. Mohapatra DP, Misonou H, Pan SJ, Held JE, Surmeier DJ, Trimmer JS. Regulation of intrinsic excitability in hippocampal neurons by activity-dependent modulation of the KV2.1 potassium channel. Channels (Austin). 2009; 3:46–56. PMID: 19276663.
Article23. Speca DJ, Ogata G, Mandikian D, Bishop HI, Wiler SW, Eum K, Wenzel HJ, Doisy ET, Matt L, Campi KL, Golub MS, Nerbonne JM, Hell JW, Trainor BC, Sack JT, Schwartzkroin PA, Trimmer JS. Deletion of the Kv2.1 delayed rectifier potassium channel leads to neuronal and behavioral hyperexcitability. Genes Brain Behav. 2014; 13:394–408. PMID: 24494598.
Article24. Wang CY, Huang AQ, Zhou MH, Mei YA. GDF15 regulates Kv2.1-mediated outward K+ current through the Akt/mTOR signalling pathway in rat cerebellar granule cells. Biochem J. 2014; 460:35–47. PMID: 24597762.25. Ito T, Nuriya M, Yasui M. Regulation of Kv2.1 phosphorylation in an animal model of anoxia. Neurobiol Dis. 2010; 38:85–91. PMID: 20079839.
Article26. Choe H, Lee YK, Lee YT, Choe H, Ko SH, Joo CU, Kim MH, Kim GS, Eun JS, Kim JH, Chae SW, Kwak YG. Papaverine blocks hKv1.5 channel current and human atrial ultrarapid delayed rectifier K+ currents. J Pharmacol Exp Ther. 2003; 304:706–712. PMID: 12538825.27. Perchenet L, Clément-Chomienne O. Characterization of mibefradil block of the human heart delayed rectifier hKv1.5. J Pharmacol Exp Ther. 2000; 295:771–778. PMID: 11046117.28. Rampe D, Wible B, Fedida D, Dage RC, Brown AM. Verapamil blocks a rapidly activating delayed rectifier K+ channel cloned from human heart. Mol Pharmacol. 1993; 44:642–648. PMID: 8371716.29. Tiran Z, Peretz A, Attali B, Elson A. Phosphorylation-dependent regulation of Kv2.1 Channel activity at tyrosine 124 by Src and by protein-tyrosine phosphatase epsilon. J Biol Chem. 2003; 278:17509–17514. PMID: 12615930.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of acidic pH on voltage-gated ion channels in rat trigeminal mesencephalic nucleus neurons
- Inhibition by Norfluoxetine, the Major Metabolite of Fluoxetine, of Voltage-Gated K+ Channels in Primary Cultured Rat Hippocampal Neurons
- Kainic Acid Treatment Increases Ca²âº-mediated Neurotoxicity in the Mouse Hippocampus
- N-acetyl-L-cysteine and cysteine increase intracellular calcium concentration in human neutrophils
- Effect of Genistein, a Tyrosine Kinase Inhibitor, on the Cloned Rat Brain Potassium Channel Kv1.5