Korean J Physiol Pharmacol.
2017 Mar;21(2):215-223. 10.4196/kjpp.2017.21.2.215.
Effects of acidic pH on voltage-gated ion channels in rat trigeminal mesencephalic nucleus neurons
- Affiliations
-
- 1Department of Pharmacology, School of Dentistry, Kyungpook National University, Daegu 41940, Korea. jis7619@knu.ac.kr
- 2Brain Science & Engineering Institute, Kyungpook National University, Daegu 41940, Korea.
- KMID: 2371040
- DOI: http://doi.org/10.4196/kjpp.2017.21.2.215
Abstract
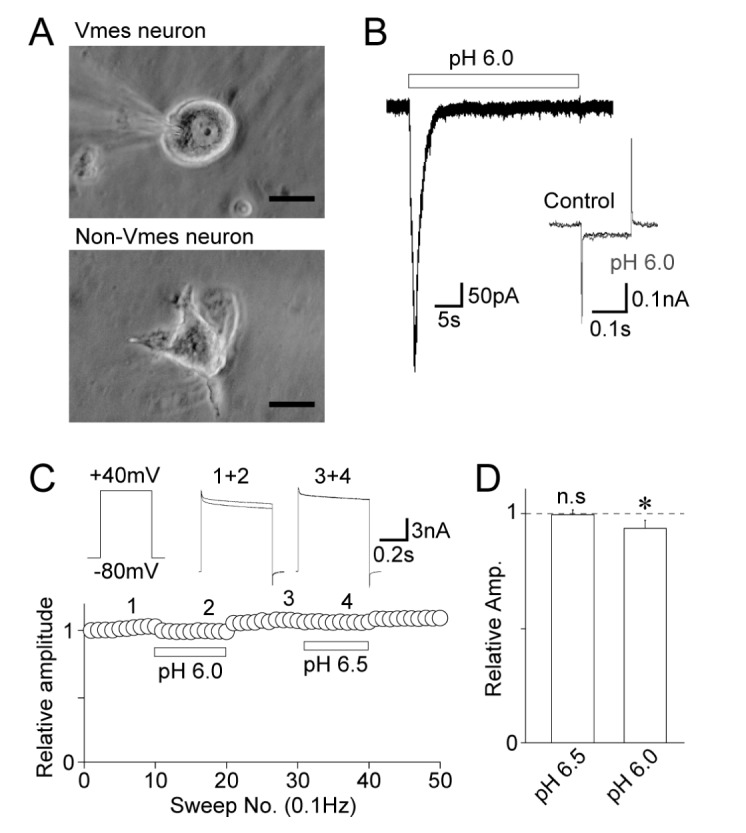
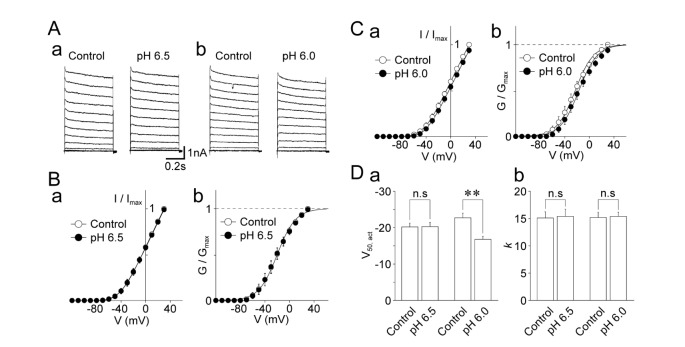
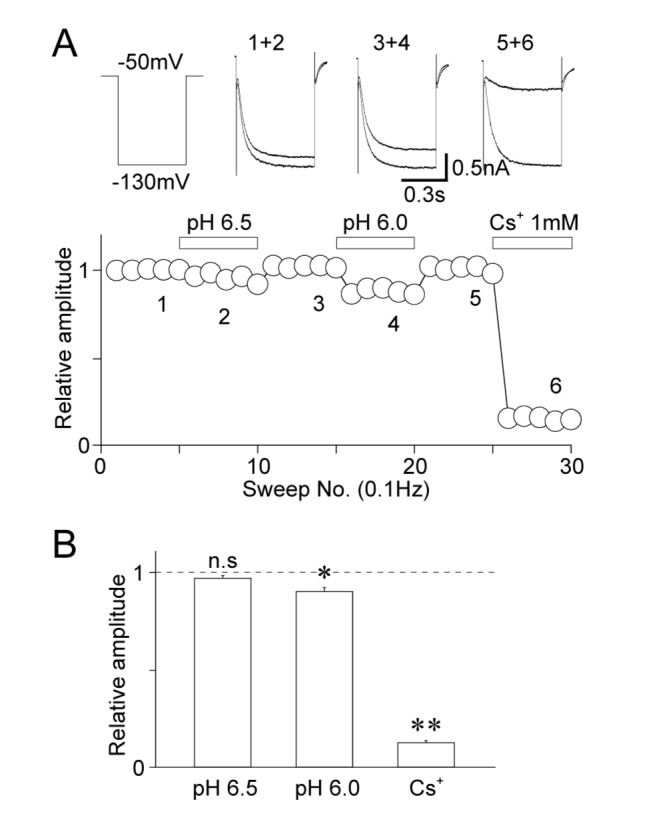
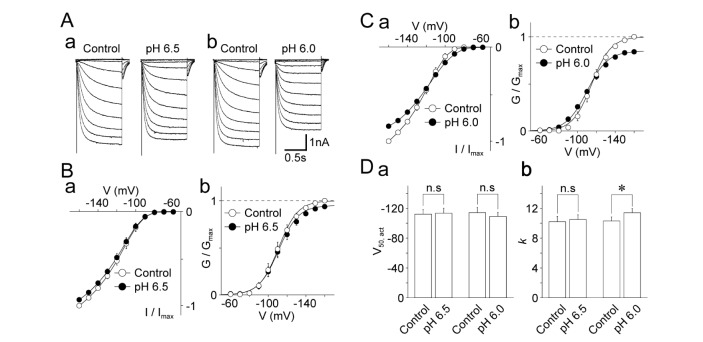
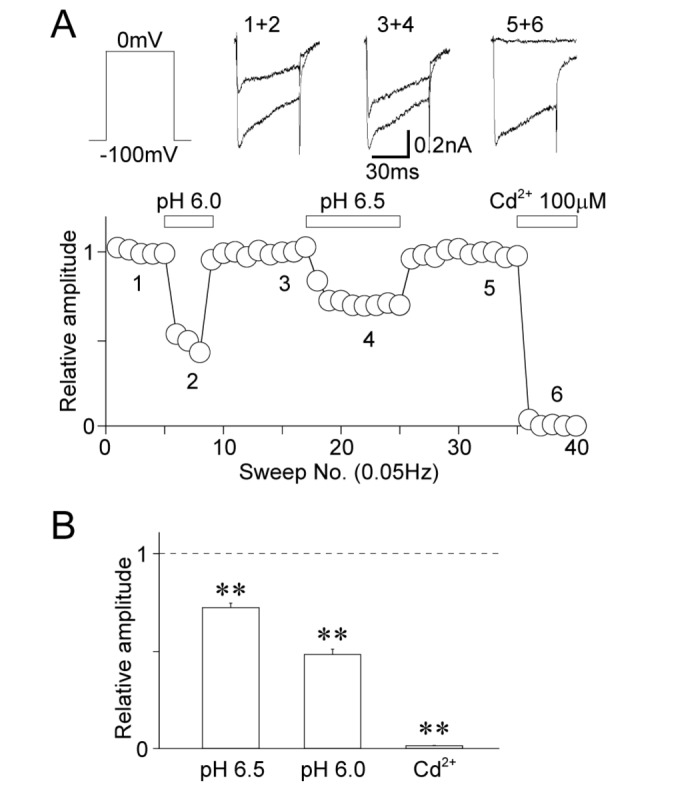
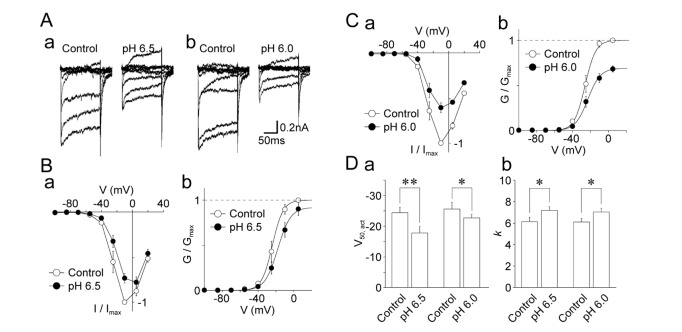
- The effects of acidic pH on several voltage-dependent ion channels, such as voltage-dependent K⺠and Ca²âº channels, and hyperpolarization-gated and cyclic nucleotide-activated cation (HCN) channels, were examined using a whole-cell patch clamp technique on mechanically isolated rat mesencephalic trigeminal nucleus neurons. The application of a pH 6.5 solution had no effect on the peak amplitude of voltage-dependent K⺠currents. A pH 6.0 solution slightly, but significantly inhibited the peak amplitude of voltage-dependent K⺠currents. The pH 6.0 also shifted both the current-voltage and conductance-voltage relationships to the depolarization range. The application of a pH 6.5 solution scarcely affected the peak amplitude of membrane currents mediated by HCN channels, which were profoundly inhibited by the general HCN channel blocker Cs⺠(1 mM). However, the pH 6.0 solution slightly, but significantly inhibited the peak amplitude of HCN-mediated currents. Although the pH 6.0 solution showed complex modulation of the current-voltage and conductance-voltage relationships, the midpoint voltages for the activation of HCN channels were not changed by acidic pH. On the other hand, voltage-dependent Ca²âº channels were significantly inhibited by an acidic pH. The application of an acidic pH solution significantly shifted the current-voltage and conductance-voltage relationships to the depolarization range. The modulation of several voltage-dependent ion channels by an acidic pH might affect the excitability of mesencephalic trigeminal nucleus neurons, and thus physiological functions mediated by the mesencephalic trigeminal nucleus could be affected in acidic pH conditions.
Keyword
MeSH Terms
Figure
Reference
-
1. Reeh PW, Steen KH. Tissue acidosis in nociception and pain. Prog Brain Res. 1996; 113:143–151. PMID: 9009732.2. Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends Neurosci. 1992; 15:396–402. PMID: 1279865.
Article3. de Hemptinne A, Marrannes R, Vanheel B. Surface pH and the control of intracellular pH in cardiac and skeletal muscle. Can J Physiol Pharmacol. 1987; 65:970–977. PMID: 3304590.4. Steen KH, Reeh PW. Sustained graded pain and hyperalgesia from harmless experimental tissue acidosis in human skin. Neurosci Lett. 1993; 154:113–116. PMID: 8361622.
Article5. Steen KH, Reeh PW, Anton F, Handwerker HO. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J Neurosci. 1992; 12:86–95. PMID: 1309578.
Article6. Steen KH, Issberner U, Reeh PW. Pain due to experimental acidosis in human skin: evidence for non-adapting nociceptor excitation. Neurosci Lett. 1995; 199:29–32. PMID: 8584219.
Article7. Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci. 2003; 26:477–483. PMID: 12948658.
Article8. Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006; 29:578–586. PMID: 16891000.
Article9. Wemmie JA, Taugher RJ, Kreple CJ. Acid-sensing ion channels in pain and disease. Nat Rev Neurosci. 2013; 14:461–471. PMID: 23783197.
Article10. Holzer P. Acid-sensitive ion channels and receptors. Handb Exp Pharmacol. 2009; (194):283–332. PMID: 19655111.
Article11. Cody FW, Lee RW, Taylor A. A functional analysis of the components of the mesencephalic nucleus of the fifth nerve in the cat. J Physiol. 1972; 226:249–261. PMID: 4263681.
Article12. Jacquin MF, Rhoades RW, Enfiejian HL, Egger MD. Organization and morphology of masticatory neurons in the rat: a retrograde HRP study. J Comp Neurol. 1983; 218:239–256. PMID: 6604076.
Article13. Lazarov NE. Comparative analysis of the chemical neuroanatomy of the mammalian trigeminal ganglion and mesencephalic trigeminal nucleus. Prog Neurobiol. 2002; 66:19–59. PMID: 11897404.
Article14. Verdier D, Lund JP, Kolta A. Synaptic inputs to trigeminal primary afferent neurons cause firing and modulate intrinsic oscillatory activity. J Neurophysiol. 2004; 92:2444–2455. PMID: 15381749.
Article15. Yokomizo Y, Murai Y, Tanaka E, Inokuchi H, Kusukawa J, Higashi H. Excitatory GABAergic synaptic potentials in the mesencephalic trigeminal nucleus of adult rat in vitro. Neurosci Res. 2005; 51:463–474. PMID: 15740809.
Article16. Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci U S A. 2001; 98:711–716. PMID: 11120882.
Article17. Lu Y, Ma X, Sabharwal R, Snitsarev V, Morgan D, Rahmouni K, Drummond HA, Whiteis CA, Costa V, Price M, Benson C, Welsh MJ, Chapleau MW, Abboud FM. The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron. 2009; 64:885–897. PMID: 20064394.
Article18. Molliver DC, Immke DC, Fierro L, Paré M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain. 2005; 1:35. PMID: 16305749.
Article19. Connor M, Naves LA, McCleskey EW. Contrasting phenotypes of putative proprioceptive and nociceptive trigeminal neurons innervating jaw muscle in rat. Mol Pain. 2005; 1:31. PMID: 16242047.
Article20. Kang IS, Cho JH, Choi IS, Kim DY, Jang IS. Acidic pH modulation of Na+ channels in trigeminal mesencephalic nucleus neurons. Neuroreport. 2016; 27:1274–1280. PMID: 27755281.21. Rhee JS, Ishibashi H, Akaike N. Calcium channels in the GABAergic presynaptic nerve terminals projecting to meynert neurons of the rat. J Neurochem. 1999; 72:800–807. PMID: 9930756.
Article22. Womble MD, Moises HC. Hyperpolarization-activated currents in neurons of the rat basolateral amygdala. J Neurophysiol. 1993; 70:2056–2065. PMID: 7507523.
Article23. Murase K, Ryu PD, Randic M. Excitatory and inhibitory amino acids and peptide-induced responses in acutely isolated rat spinal dorsal horn neurons. Neurosci Lett. 1989; 103:56–63. PMID: 2476693.
Article24. Nakamura M, Jang IS. Characterization of proton-induced currents in rat trigeminal mesencephalic nucleus neurons. Brain Res. 2014; 1583:12–22. PMID: 25128599.
Article25. Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stühmer W, Wang X. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev. 2005; 57:473–508. PMID: 16382104.
Article26. Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011; 164(Suppl 1):S1–S324. PMID: 22040146.
Article27. Aguilar-Bryan L, Clement JP 4th, Gonzalez G, Kunjilwar K, Babenko A, Bryan J. Toward understanding the assembly and structure of KATP channels. Physiol Rev. 1998; 78:227–245. PMID: 9457174.
Article28. Kobayashi T, Ikeda K. G protein-activated inwardly rectifying potassium channels as potential therapeutic targets. Curr Pharm Des. 2006; 12:4513–4523. PMID: 17168757.
Article29. Kim D. Physiology and pharmacology of two-pore domain potassium channels. Curr Pharm Des. 2005; 11:2717–2736. PMID: 16101451.
Article30. Goldstein SA, Bayliss DA, Kim D, Lesage F, Plant LD, Rajan S. International Union of Pharmacology. LV. Nomenclature and molecular relationships of two-P potassium channels. Pharmacol Rev. 2005; 57:527–540. PMID: 16382106.
Article31. Matsumoto S, Yoshida S, Takahashi M, Saiki C, Takeda M. The roles of I(D), I(A) and I(K) in the electrophysiological functions of small diameter rat trigeminal ganglion neurons. Curr Mol Pharmacol. 2010; 3:30–36. PMID: 20030627.32. Tombaugh GC, Somjen GG. Effects of extracellular pH on voltage-gated Na+, K+ and Ca2+ currents in isolated rat CA1 neurons. J Physiol. 1996; 493:719–732. PMID: 8799894.33. Postea O, Biel M. Exploring HCN channels as novel drug targets. Nat Rev Drug Discov. 2011; 10:903–914. PMID: 22094868.
Article34. He C, Chen F, Li B, Hu Z. Neurophysiology of HCN channels: from cellular functions to multiple regulations. Prog Neurobiol. 2014; 112:1–23. PMID: 24184323.
Article35. Cohen IS, Robinson RB. Pacemaker current and automatic rhythms: toward a molecular understanding. Handb Exp Pharmacol. 2006; (171):41–71.
Article36. Siu CW, Lieu DK, Li RA. HCN-encoded pacemaker channels: from physiology and biophysics to bioengineering. J Membr Biol. 2006; 214:115–122. PMID: 17558529.
Article37. Wahl-Schott C, Fenske S, Biel M. HCN channels: new roles in sinoatrial node function. Curr Opin Pharmacol. 2014; 15:83–90. PMID: 24441197.
Article38. Dunlop J, Vasilyev D, Lu P, Cummons T, Bowlby MR. Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels and pain. Curr Pharm Des. 2009; 15:1767–1772. PMID: 19442189.
Article39. Noam Y, Bernard C, Baram TZ. Towards an integrated view of HCN channel role in epilepsy. Curr Opin Neurobiol. 2011; 21:873–879. PMID: 21782415.
Article40. Emery EC, Young GT, McNaughton PA. HCN2 ion channels: an emerging role as the pacemakers of pain. Trends Pharmacol Sci. 2012; 33:456–463. PMID: 22613784.
Article41. DiFrancesco JC, DiFrancesco D. Dysfunctional HCN ion channels in neurological diseases. Front Cell Neurosci. 2015; 6:174. PMID: 25805968.
Article42. Khakh BS, Henderson G. Hyperpolarization-activated cationic currents (Ih) in neurones of the trigeminal mesencephalic nucleus of the rat. J Physiol. 1998; 510:695–704. PMID: 9660886.
Article43. Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev. 2015; 67:821–870. PMID: 26362469.
Article44. Wu LG, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997; 20:204–212. PMID: 9141196.
Article45. Lacinová L. Pharmacology of recombinant low-voltage activated calcium channels. Curr Drug Targets CNS Neurol Disord. 2004; 3:105–111. PMID: 15078185.46. Ohmori H, Yoshii M. Surface potential reflected in both gating and permeation mechanisms of sodium and calcium channels of the tunicate egg cell membrane. J Physiol. 1977; 267:429–463. PMID: 17734.
Article47. Irisawa H, Sato R. Intra- and extracellular actions of proton on the calcium current of isolated guinea pig ventricular cells. Circ Res. 1986; 59:348–355. PMID: 2429781.
Article48. Tytgat J, Nilius B, Carmeliet E. Modulation of the T-type cardiac Ca channel by changes in proton concentration. J Gen Physiol. 1990; 96:973–990. PMID: 2177772.
Article49. Hille B. Charges and potentials at the nerve surface. Divalent ions and pH. J Gen Physiol. 1968; 51:221–236.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Calcium channels
- Distribution of ion channels in trigeminal ganglion neuron of rat
- Gabapentin Attenuates the Activation of Transient Receptor Potential A1 by Cinnamaldehyde
- The effects of pain-producing agents on the voltage-dependent ionic channels of rat trigeminal ganglion
- Localization of Nerves Innervating the Sublingual Gland in the Rat Brain Using Pseudorabies Virus