Korean J Physiol Pharmacol.
2017 Mar;21(2):251-257. 10.4196/kjpp.2017.21.2.251.
Inhibition of K⺠outward currents by linopirdine in the cochlear outer hair cells of circling mice within the first postnatal week
- Affiliations
-
- 1Department of Physiology, College of Medicine, Dankook University, Cheonan 31116, Korea. ansil67@hanmail.net
- KMID: 2371044
- DOI: http://doi.org/10.4196/kjpp.2017.21.2.251
Abstract
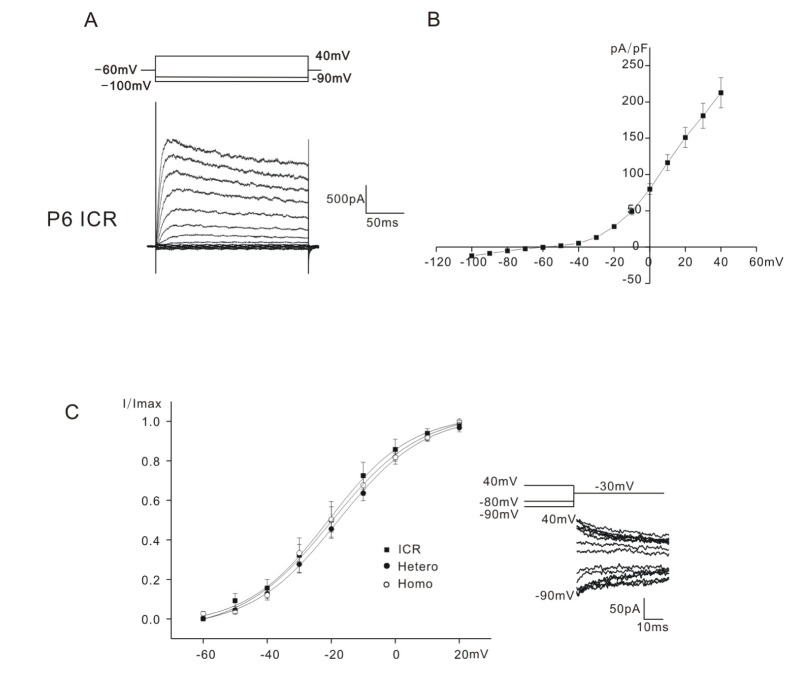
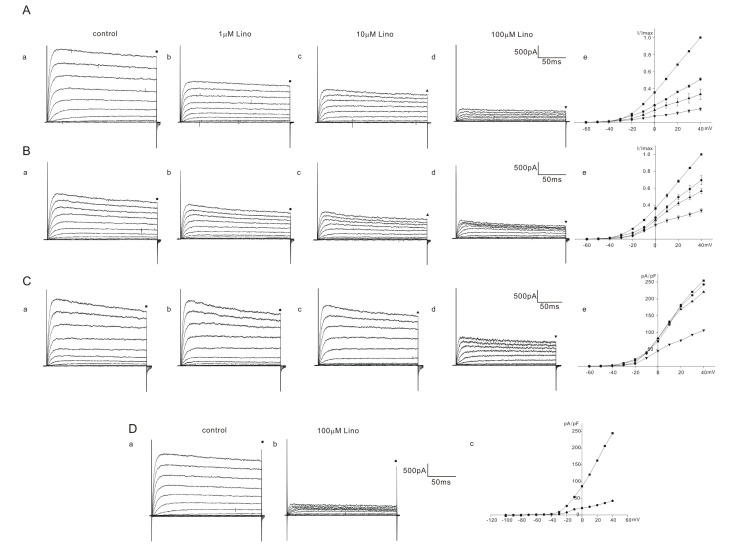
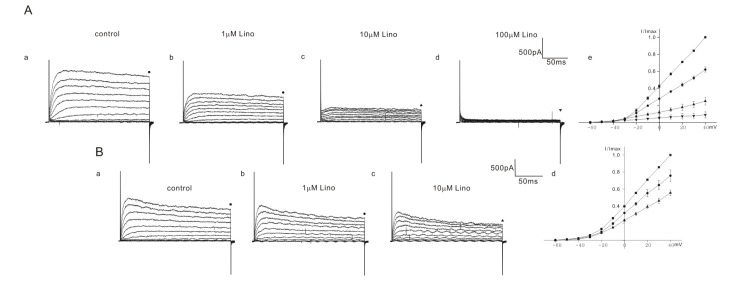
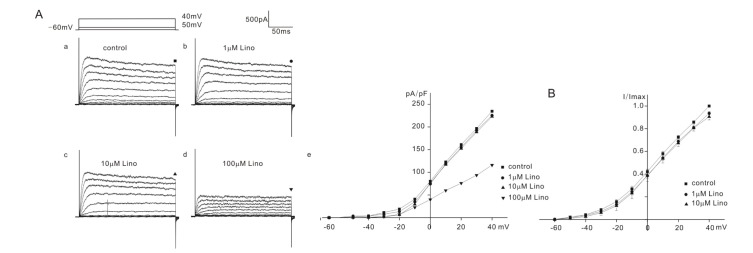
- Inhibition of K⺠outward currents by linopirdine in the outer hair cells (OHCs) of circling mice (homozygous (cir/cir) mice), an animal model for human deafness (DFNB6 type), was investigated using a whole cell patch clamp technique. Littermate heterozygous (+/cir) and ICR mice of the same age (postnatal day (P) 0 -P6) were used as controls. Voltage steps from -100 mV to 40 mV elicited small inward currents (-100 mV~-70 mV) and slow rising K⺠outward currents (-60 mV ~40 mV) which activated near -50 mV in all OHCs tested. Linopirdine, a known blocker of K⺠currents activated at negative potentials (I(K,n)), did cause inhibition at varying degree (severe, moderate, mild) in K⺠outward currents of heterozygous (+/cir) or homozygous (cir/cir) mice OHCs in the concentration range between 1 and 100 µM, while it was apparent only in one ICR mice OHC out of nine OHCs at 100 µM. Although the half inhibition concentrations in heterozygous (+/cir) or homozygous (cir/cir) mice OHCs were close to those reported in I(K,n), biophysical and pharmacological properties of K⺠outward currents, such as the activation close to -50 mV, small inward currents evoked by hyperpolarizing steps and TEA sensitivity, were not in line with I(K,n) reported in other tissues. Our results show that the delayed rectifier type K⺠outward currents, which are not similar to I(K,n) with respect to biophysical and pharmacological properties, are inhibited by linopirdine in the developing (P0~P6) homozygous (cir/cir) or heterozygous (+/cir) mice OHCs.
Keyword
MeSH Terms
Figure
Reference
-
1. Marcotti W, Johnson SL, Holley MC, Kros CJ. Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. J Physiol. 2003; 548:383–400. PMID: 12588897.
Article2. Marcotti W, Géléoc GS, Lennan GW, Kros CJ. Transient expression of an inwardly rectifying potassium conductance in developing inner and outer hair cells along the mouse cochlea. Pflugers Arch. 1999; 439:113–122. PMID: 10651007.
Article3. Dulon D, Luo L, Zhang C, Ryan AF. Expression of small-conductance calcium-activated potassium channels (SK) in outer hair cells of the rat cochlea. Eur J Neurosci. 1998; 10:907–915. PMID: 9753158.
Article4. Roux I, Wersinger E, McIntosh JM, Fuchs PA, Glowatzki E. Onset of cholinergic efferent synaptic function in sensory hair cells of the rat cochlea. J Neurosci. 2011; 31:15092–15101. PMID: 22016543.
Article5. Marcotti W, Kros CJ. Developmental expression of the potassium current IK,n contributes to maturation of mouse outer hair cells. J Physiol. 1999; 520:653–660. PMID: 10545133.6. Kros CJ, Ruppersberg JP, Rüsch A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature. 1998; 394:281–284. PMID: 9685158.
Article7. Ahn JW, Kang SW, Ahn SC. Characteristics of K+ outward currents in the cochlear outer hair cells of circling mice within the first postnatal week. Korean J Physiol Pharmacol. 2015; 19:383–388. PMID: 26170743.8. Chung WH, Kim KR, Cho YS, Cho DY, Woo JH, Ryoo ZY, Cho KI, Hong SH. Cochlear pathology of the circling mouse: a new mouse model of DFNB6. Acta Otolaryngol. 2007; 127:244–251. PMID: 17364360.
Article9. Lee JW, Lee EJ, Hong SH, Chung WH, Lee HT, Lee TW, Lee JR, Kim HT, Suh JG, Kim TY, Ryoo ZY. Circling mouse: possible animal model for deafness. Comp Med. 2001; 51:550–554. PMID: 11924819.10. Lee Y, Chang SY, Jung JY, Ahn SC. Reinvestigation of cochlear pathology in circling mice. Neurosci Lett. 2015; 594:30–35. PMID: 25817368.
Article11. Hong SH, Kim MJ, Ahn SC. Glutamatergic transmission is sustained at a later period of development of medial nucleus of the trapezoid body-lateral superior olive synapses in circling mice. J Neurosci. 2008; 28:13003–13007. PMID: 19036993.
Article12. Helyer RJ, Kennedy HJ, Davies D, Holley MC, Kros CJ. Development of outward potassium currents in inner and outer hair cells from the embryonic mouse cochlea. Audiol Neurootol. 2005; 10:22–34. PMID: 15486441.
Article13. Pattnaik BR, Hughes BA. Effects of KCNQ channel modulators on the M-type potassium current in primate retinal pigment epithelium. Am J Physiol Cell Physiol. 2012; 302:C821–C833. PMID: 22135213.
Article14. Søgaard R, Ljungstrøm T, Pedersen KA, Olesen SP, Jensen BS. KCNQ4 channels expressed in mammalian cells: functional characteristics and pharmacology. Am J Physiol Cell Physiol. 2001; 280:C859–C866. PMID: 11245603.
Article15. Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998; 282:1890–1893. PMID: 9836639.
Article16. Schwarz JR, Glassmeier G, Cooper EC, Kao TC, Nodera H, Tabuena D, Kaji R, Bostock H. KCNQ channels mediate IKs, a slow K+ current regulating excitability in the rat node of Ranvier. J Physiol. 2006; 573:17–34. PMID: 16527853.17. Housley GD, Ashmore JF. Ionic currents of outer hair cells isolated from the guinea-pig cochlea. J Physiol. 1992; 448:73–98. PMID: 1593487.
Article18. Kimitsuki T, Komune N, Noda T, Takaiwa K, Ohashi M, Komune S. Property of IK,n in inner hair cells isolated from guinea-pig cochlea. Hear Res. 2010; 261:57–62. PMID: 20060884.19. Bal M, Zhang J, Zaika O, Hernandez CC, Shapiro MS. Homomeric and heteromeric assembly of KCNQ (Kv7) K+ channels assayed by total internal reflection fluorescence/fluorescence resonance energy transfer and patch clamp analysis. J Biol Chem. 2008; 283:30668–30676. PMID: 18786918.20. Robbins J. KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacol Ther. 2001; 90:1–19. PMID: 11448722.
Article21. Schnee ME, Brown BS. Selectivity of linopirdine (DuP 996), a neurotransmitter release enhancer, in blocking voltage-dependent and calcium-activated potassium currents in hippocampal neurons. J Pharmacol Exp Ther. 1998; 286:709–717. PMID: 9694925.22. Lamas JA, Selyanko AA, Brown DA. Effects of a cognition-enhancer, linopirdine (DuP 996), on M-type potassium currents (IK(M)) and some other voltage- and ligand-gated membrane currents in rat sympathetic neurons. Eur J Neurosci. 1997; 9:605–616. PMID: 9104602.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characteristics of K+ Outward Currents in the Cochlear Outer Hair Cells of Circling Mice within the First Postnatal Week
- Over-expression of myosin7A in cochlear hair cells of circling mice
- Temperature Enhances Activation and Inactivation Kinetics of Potassium Currents in Inner Hair Cells Isolated from Guinea-Pig Cochlea
- Mechanism of Efferent Inhibition in Cochlear Hair Cell
- Changes of Cochlear Nerve Terminals after Temporary Noise-Induced Hearing Loss