Clin Exp Otorhinolaryngol.
2013 Sep;6(3):140-145.
Temperature Enhances Activation and Inactivation Kinetics of Potassium Currents in Inner Hair Cells Isolated from Guinea-Pig Cochlea
- Affiliations
-
- 1Department of Otolaryngology, Kyushu Central Hospital, Fukuoka, Japan. kimitsuki@kyushu-ctr-hsp.com
- 2Department of Otolaryngology, Graduate School of Medical Sciences, Kyushu University Faculty of Medicine, Fukuoka, Japan.
Abstract
OBJECTIVES
Until recently, most patch-clamp recordings in inner hair cells (IHCs) have been performed at room temperature. The results acquired at room temperature should be corrected if they are to be related to in vivo findings. However, the temperature dependency to ion channels in IHCs is unknown. The aim of this study was to investigate the effect of temperature on the potassium currents in IHCs.
METHODS
IHCs were isolated from a mature guinea-pig cochlea and potassium currents were recorded at room temperature (around 25degrees C) and physiological temperatures (35degrees C-37degrees C).
RESULTS
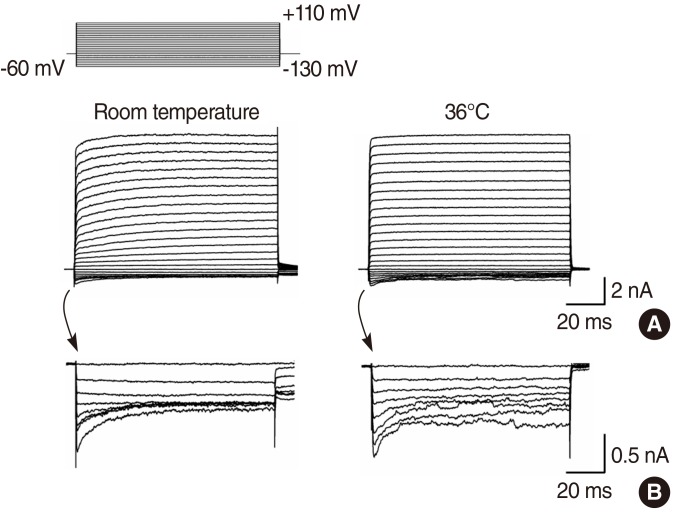
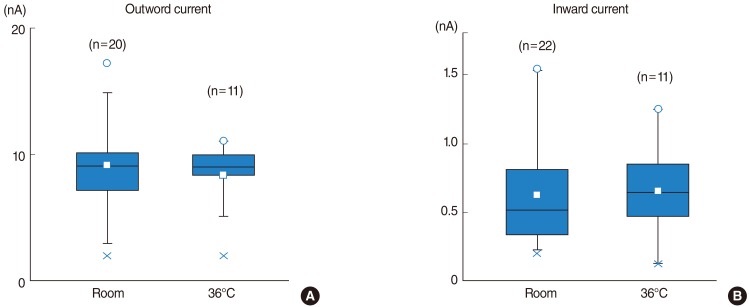
IHCs showed outwardly rectifying currents in response to depolarizing voltage pulses, with only a slight inward current when hyperpolarized. The amplitude of both outward and inward currents demonstrated no temperature dependency, however, activation and inactivation rates were faster at 36degrees C than at room temperature. Half-time for activation was shorter at 36degrees C than at room temperature at membrane potentials of -10, +10, +20, +30, and +40 mV. Q10 for the activation rate was 1.83. The inactivation time constant in outward tetraethylammonium-sensitive potassium currents was much smaller at 36degrees C than at room temperature between the membrane potentials of -20 and +60 mV. Q10 for the inactivation time constant was 3.19.
CONCLUSION
The results of this study suggest that the amplitude of potassium currents in IHCs showed no temperature dependence either in outward or inward-going currents, however, activation and inactivation accelerated at physiological temperatures.
Keyword
MeSH Terms
Figure
Reference
-
1. Kros CJ, Crawford AC. Potassium currents in inner hair cells isolated from the guinea-pig cochlea. J Physiol. 1990; 2. 421:263–291. PMID: 2348394.
Article2. Marcotti W, Johnson SL, Holley MC, Kros CJ. Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. J Physiol. 2003; 4. 548(Pt 2):383–400. PMID: 12588897.
Article3. Kimitsuki T, Komune N, Noda T, Takaiwa K, Ohashi M, Komune S. Property of Ik, n in inner hair cells isolated from guinea-pig cochlea. Hear Res. 2010; 3. 261(1-2):57–62. PMID: 20060884.4. Fernandez-Alfonso T, Ryan TA. The kinetics of synaptic vesicle pool depletion at CNS synaptic terminals. Neuron. 2004; 3. 41(6):943–953. PMID: 15046726.
Article5. Micheva KD, Smith SJ. Strong effects of subphysiological temperature on the function and plasticity of mammalian presynaptic terminals. J Neurosci. 2005; 8. 25(33):7481–7488. PMID: 16107635.
Article6. Nouvian R. Temperature enhances exocytosis efficiency at the mouse inner hair cell ribbon synapse. J Physiol. 2007; 10. 584(Pt 2):535–542. PMID: 17717016.
Article7. Sanz AG, Hospital S, Badia A, Clos MV. Presynaptic effect of 7-OH-DPAT on evoked [3H]-acetylcholine release in rat striatal synaptosomes. Brain Res. 2000; 8. 874(2):116–122. PMID: 10960595.
Article8. Sumners C, Fleegal MA, Zhu M. Angiotensin AT1 receptor signalling pathways in neurons. Clin Exp Pharmacol Physiol. 2002; May-Jun. 29(5-6):483–490. PMID: 12010196.
Article9. He DZ, Zheng J, Edge R, Dallos P. Isolation of cochlear inner hair cells. Hear Res. 2000; 7. 145(1-2):156–160. PMID: 10867288.
Article10. Santos-Sacchi J, Huang GJ, Wu M. Mapping the distribution of outer hair cell voltage-dependent conductances by electrical amputation. Biophys J. 1997; 9. 73(3):1424–1429. PMID: 9284309.
Article11. Hille B. Potassium channels and chloride channels. In : Hille B, editor. Ionic channels of excitable membranes. 2nd ed. Sunderland, MA: Sinauer;1991. p. 115–139.12. Kimitsuki T, Ohashi M, Wada Y, Fukudome S, Komune S. Inactivating and non-inactivating potassium currents in isolated inner hair cells from guinea pig cochlea. Acta Otolaryngol Suppl. 2004; 8. (553):28–32. PMID: 15277032.
Article13. Mitsuiye T, Shinagawa Y, Noma A. Temperature dependence of the inward rectifier K+ channel gating in guinea-pig ventricular cells. Jpn J Physiol. 1997; 2. 47(1):73–79. PMID: 9159645.14. Beam KG, Donaldson PL. A quantitative study of potassium channel kinetics in rat skeletal muscle from 1 to 37 degrees C. J Gen Physiol. 1983; 4. 81(4):485–512. PMID: 6304231.
Article15. Pahapill PA, Schlichter LC. Modulation of potassium channels in human T lymphocytes: effects of temperature. J Physiol. 1990; 3. 422:103–126. PMID: 2352174.
Article16. Brandt A, Khimich D, Moser T. Few CaV1.3 channels regulate the exocytosis of a synaptic vesicle at the hair cell ribbon synapse. J Neurosci. 2005; 12. 25(50):11577–11585. PMID: 16354915.
Article17. Johnson SL, Marcotti W. Biophysical properties of CaV1.3 calcium channels in gerbil inner hair cells. J Physiol. 2008; 2. 586(4):1029–1042. PMID: 18174213.
Article18. Kros CJ, Ruppersberg JP, Rusch A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature. 1998; 7. 394(6690):281–284. PMID: 9685158.
Article19. Bregestovski P, Redkozubov A, Alexeev A. Elevation of intracellular calcium reduces voltage-dependent potassium conductance in human T cells. Nature. 1986; Feb-Mar. 319(6056):776–778. PMID: 2419761.
Article20. Choquet D, Sarthou P, Primi D, Cazenave PA, Korn H. Cyclic AMPmodulated potassium channels in murine B cells and their precursors. Science. 1987; 3. 235(4793):1211–1214. PMID: 2434998.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Potassium Currents in Isolated Deiters' Cells of Guinea Pig
- Effects of Aminoglycoside Antibiotics on Acetylcholine-induced Potassium Currents in Guinea-pig Outer Hair Cell
- The expression of voltage-dependent K+ channels in stria vascularis of the guinea pig cochlea
- Outward Rectifying Current in Isolated Deiters' Cells from Guinea Pig Cochlea
- The effect of carbamylcholine on the depolarization-induced change of intracellular calcium in the outer hair cells of guinea pig cochlea