Korean J Physiol Pharmacol.
2015 Jul;19(4):383-388. 10.4196/kjpp.2015.19.4.383.
Characteristics of K+ Outward Currents in the Cochlear Outer Hair Cells of Circling Mice within the First Postnatal Week
- Affiliations
-
- 1Department of Physiology, College of Medicine, Dankook University, Cheonan 330-714, Korea. ansil67@hanmail.net
- KMID: 2285582
- DOI: http://doi.org/10.4196/kjpp.2015.19.4.383
Abstract
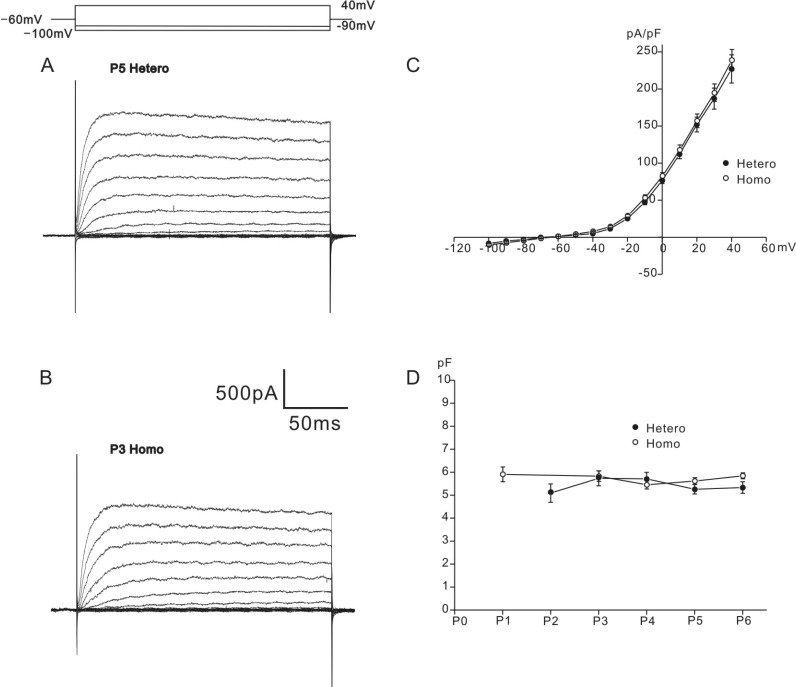
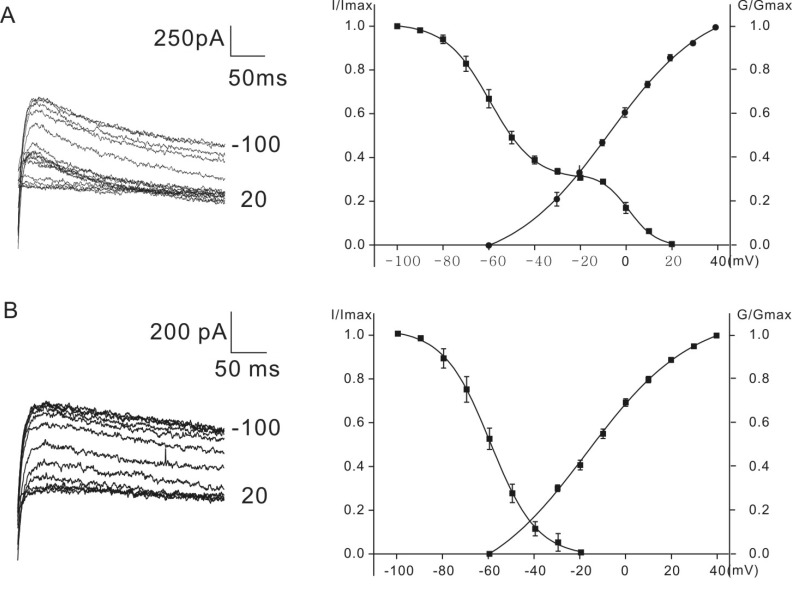
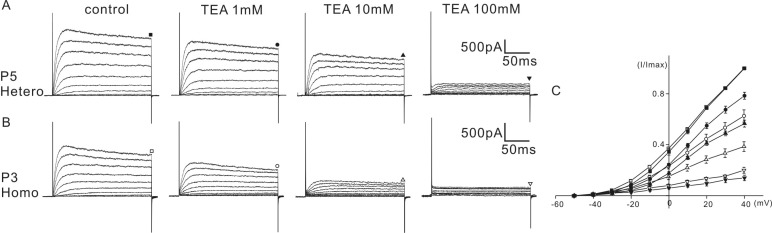
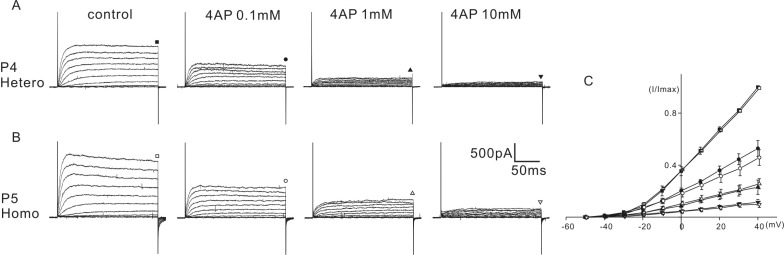
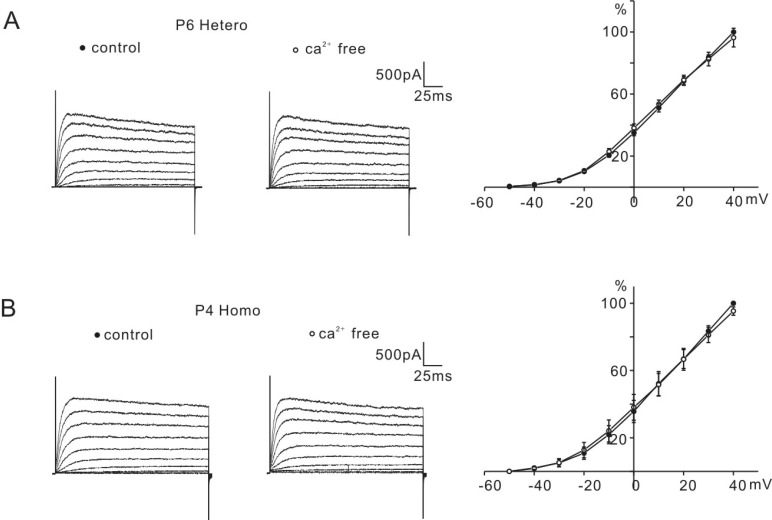
- K+ outward currents in the outer hair cells (OHCs) of circling mice (homozygous (cir/cir) mice), an animal model for human deafness (DFNB6 type), were investigated using a whole cell patch clamp technique. Littermate heterozygous (+/cir) mice of the same age (postnatal day (P) 0 -P6) were used as controls. Similar slow rising K+ currents were observed in both genotypes, but their biophysical and pharmacological properties were quite different. The values of V(half) for activation were significantly different in the heterozygous (+/cir) and homozygous (cir/cir) mice (-8.1+/-2.2 mV, heterozygous (+/cir) mice (n=7) and -17.2+/-4.2 mV, homozygous (cir/cir) mice (n=5)). The inactivation curve was expressed by a single first order Boltzmann equation in the homozygous (cir/cir) mice, while it was expressed by a sum of two first order Boltzmann equations in the heterozygous (+/cir) mice. The K+ current of homozygous (cir/cir) mice was more sensitive to TEA in the 1 to 10 mM range, while the 4-AP sensitivities were not different between the two genotypes. Removal of external Ca2+ did not affect the K+ currents in either genotype, indicating that the higher sensitivity of K+ current to TEA in the homozygous (cir/cir) mice was not due to an early expression of Ca2+ activated K+ channels. Our results suggest that the K+ outward current of developing homozygous (cir/cir) mice OHCs is different in both biophysical and pharmacological aspects than that of heterozygous (+/cir) mice.
Keyword
MeSH Terms
Figure
Reference
-
1. Marcotti W, Johnson SL, Holley MC, Kros CJ. Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. J Physiol. 2003; 548:383–400. PMID: 12588897.
Article2. Marcotti W, Géléoc GS, Lennan GW, Kros CJ. Transient expression of an inwardly rectifying potassium conductance in developing inner and outer hair cells along the mouse cochlea. Pflugers Arch. 1999; 439:113–122. PMID: 10651007.
Article3. Marcotti W, Kros CJ. Developmental expression of the potassium current IK,n contributes to maturation of mouse outer hair cells. J Physiol. 1999; 520 Pt 3:653–660. PMID: 10545133.4. Kros CJ, Ruppersberg JP, Rüsch A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature. 1998; 394:281–284. PMID: 9685158.
Article5. Lee JW, Lee EJ, Hong SH, Chung WH, Lee HT, Lee TW, Lee JR, Kim HT, Suh JG, Kim TY, Ryoo ZY. Circling mouse: possible animal model for deafness. Comp Med. 2001; 51:550–554. PMID: 11924819.6. Chung WH, Kim KR, Cho YS, Cho DY, Woo JH, Ryoo ZY, Cho KI, Hong SH. Cochlear pathology of the circling mouse: a new mouse model of DFNB6. Acta Otolaryngol. 2007; 127:244–251. PMID: 17364360.
Article7. Kharkovets T, Dedek K, Maier H, Schweizer M, Khimich D, Nouvian R, Vardanyan V, Leuwer R, Moser T, Jentsch TJ. Mice with altered KCNQ4 K+ channels implicate sensory outer hair cells in human progressive deafness. EMBO J. 2006; 25:642–652. PMID: 16437162.8. Hong SH, Kim MJ, Ahn SC. Glutamatergic transmission is sustained at a later period of development of medial nucleus of the trapezoid body-lateral superior olive synapses in circling mice. J Neurosci. 2008; 28:13003–13007. PMID: 19036993.
Article9. Helyer RJ, Kennedy HJ, Davies D, Holley MC, Kros CJ. Development of outward potassium currents in inner and outer hair cells from the embryonic mouse cochlea. Audiol Neurootol. 2005; 10:22–34. PMID: 15486441.
Article10. Mammano F, Ashmore JF. Differential expression of outer hair cell potassium currents in the isolated cochlea of the guinea-pig. J Physiol. 1996; 496:639–646. PMID: 8930832.
Article11. Liang GH, Järlebark L, Ulfendahl M, Moore EJ. Mercury Hg2+ suppression of potassium currents of outer hair cells. Neurotoxicol Teratol. 2003; 25:349–359. PMID: 12757831.12. Marcotti W, Johnson SL, Kros CJ. A transiently expressed SK current sustains and modulates action potential activity in immature mouse inner hair cells. J Physiol. 2004; 560:691–708. PMID: 15331671.
Article13. Sah P. Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996; 19:150–154. PMID: 8658599.14. Marcotti W, Johnson SL, Kros CJ. Effects of intracellular stores and extracellular Ca2+ on Ca2+-activated K+ currents in mature mouse inner hair cells. J Physiol. 2004; 557:613–633. PMID: 15064328.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of K⺠outward currents by linopirdine in the cochlear outer hair cells of circling mice within the first postnatal week
- Over-expression of myosin7A in cochlear hair cells of circling mice
- Temperature Enhances Activation and Inactivation Kinetics of Potassium Currents in Inner Hair Cells Isolated from Guinea-Pig Cochlea
- Changes of Cochlear Nerve Terminals after Temporary Noise-Induced Hearing Loss
- Prestin and Motility of the Cochlear Outer Hair Cell