Korean J Physiol Pharmacol.
2017 Mar;21(2):179-188. 10.4196/kjpp.2017.21.2.179.
Activation of autophagy at cerebral cortex and apoptosis at brainstem are differential responses to 835 MHz RF-EMF exposure
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Dankook University, Cheonan 31116, Korea. hrkim@dankook.ac.kr
- KMID: 2371036
- DOI: http://doi.org/10.4196/kjpp.2017.21.2.179
Abstract
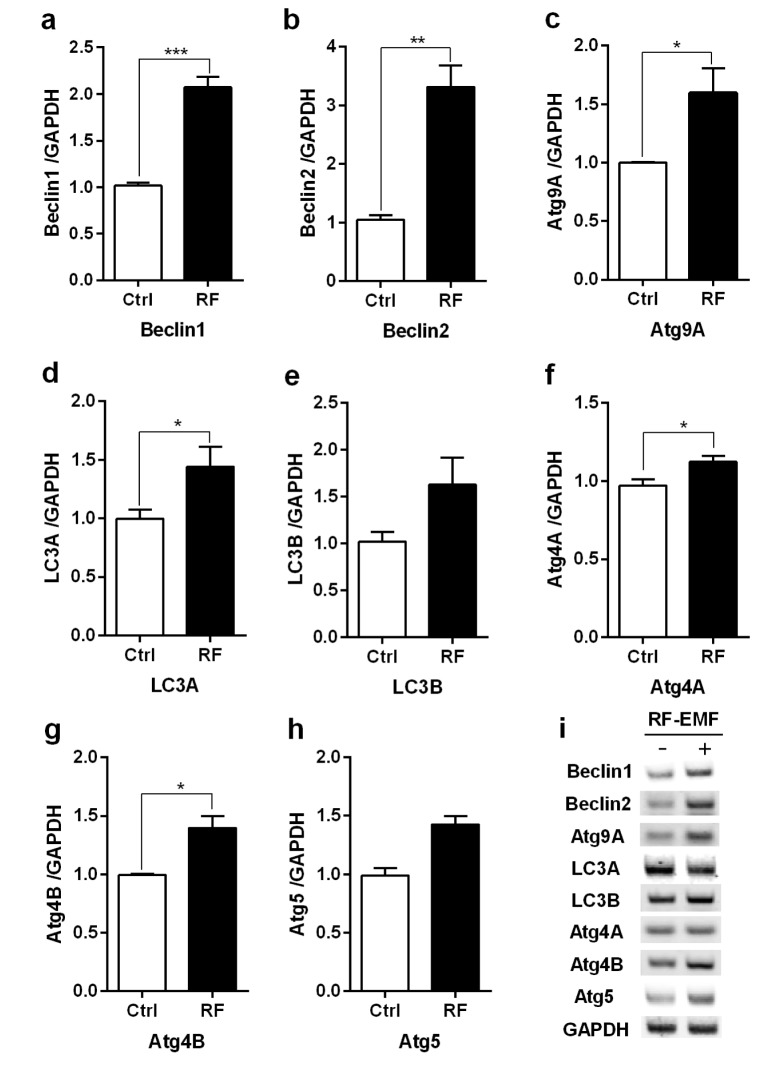
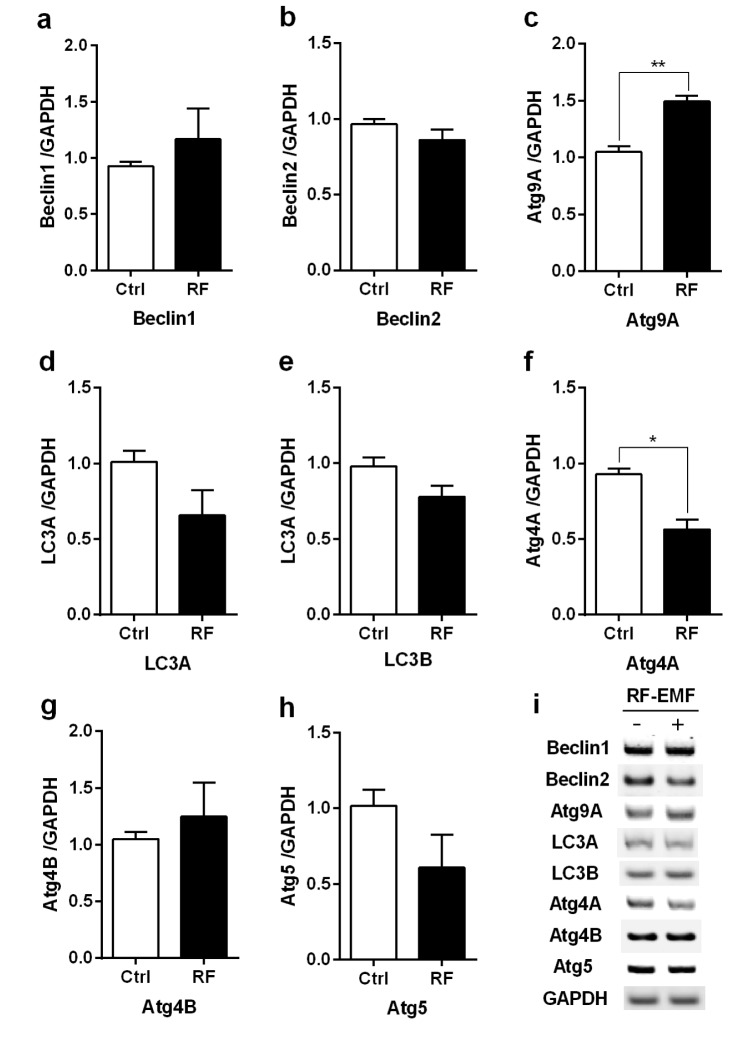
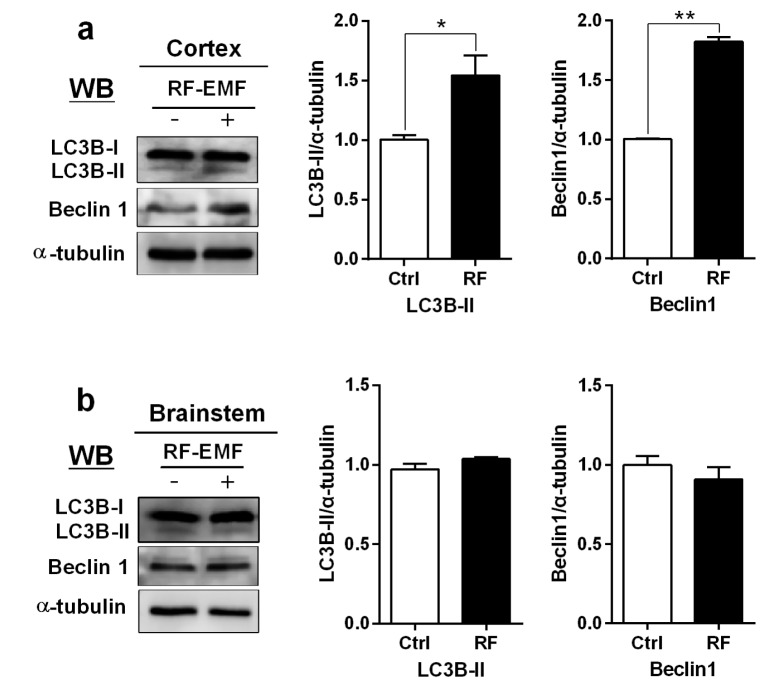
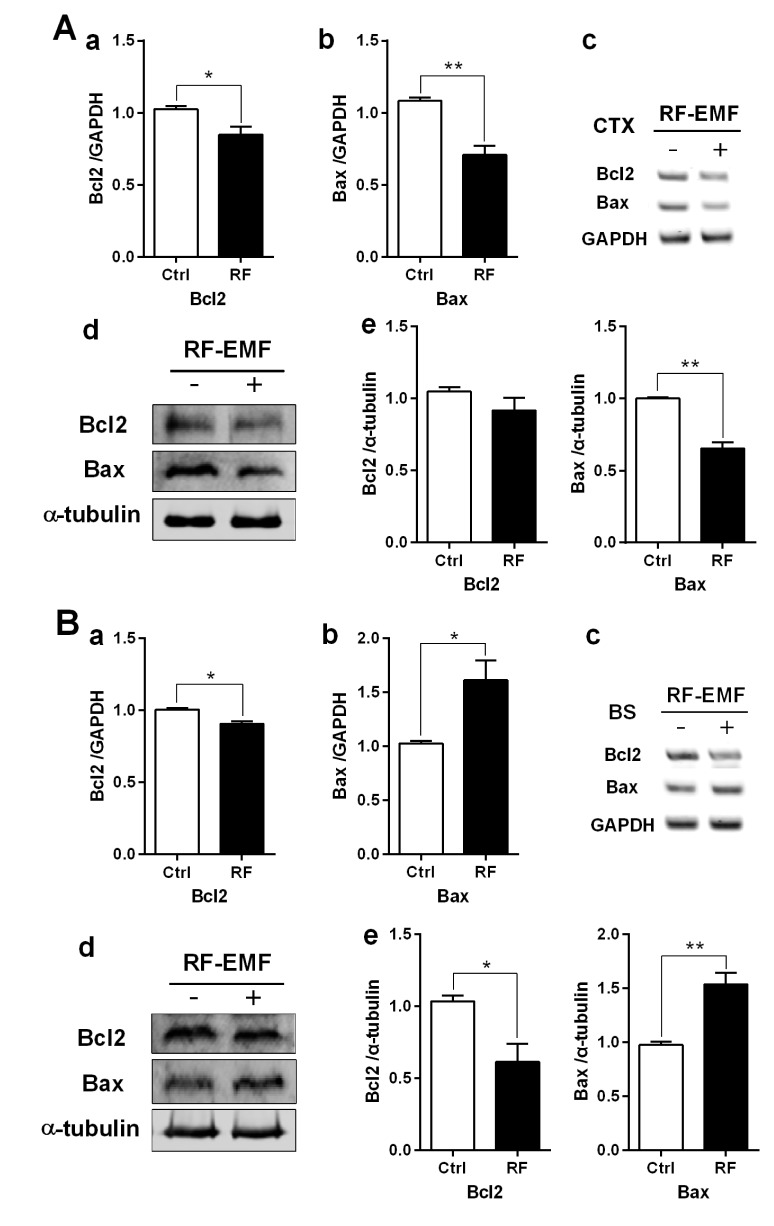
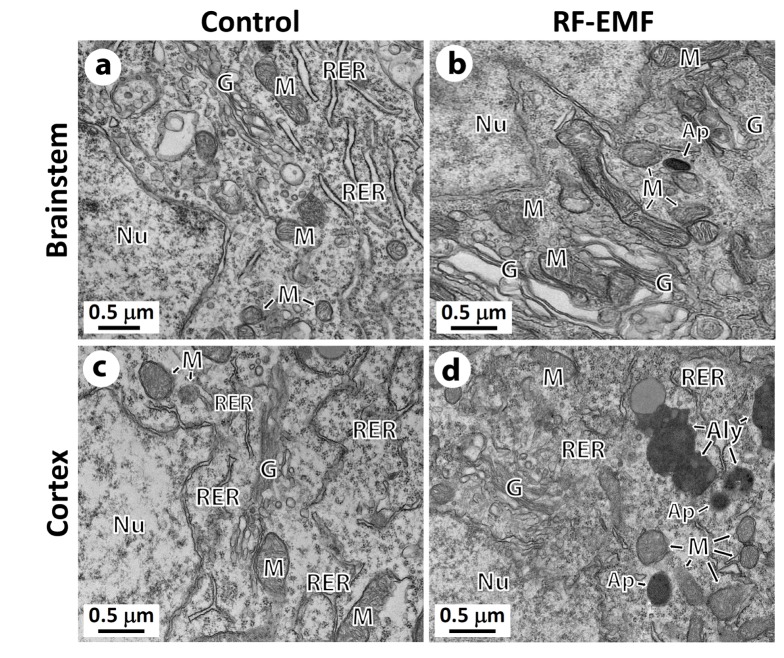
- With the explosive increase in exposure to radiofrequency electromagnetic fields (RF-EMF) emitted by mobile phones, public concerns have grown over the last few decades with regard to the potential effects of EMF exposure on the nervous system in the brain. Many researchers have suggested that RF-EMFs can effect diverse neuronal alterations in the brain, thereby affecting neuronal functions as well as behavior. Previously, we showed that long-term exposure to 835 MHz RF-EMF induces autophagy in the mice brain. In this study, we explore whether short-term exposure to RF-EMF leads to the autophagy pathway in the cerebral cortex and brainstem at 835 MHz with a specific absorption rate (SAR) of 4.0 W/kg for 4 weeks. Increased levels of autophagy genes and proteins such as LC3B-II and Beclin1 were demonstrated and the accumulation of autophagosomes and autolysosomes was observed in cortical neurons whereas apoptosis pathways were up-regulated in the brainstem but not in the cortex following 4 weeks of RF exposure. Taken together, the present study indicates that monthly exposure to RF-EMF induces autophagy in the cerebral cortex and suggests that autophagic degradation in cortical neurons against a stress of 835 MHz RF during 4 weeks could correspond to adaptation to the RF stress environment. However, activation of apoptosis rather than autophagy in the brainstem is suggesting the differential responses to the RF-EMF stresses in the brain system.
Keyword
MeSH Terms
Figure
Reference
-
1. Liu YX, Tai JL, Li GQ, Zhang ZW, Xue JH, Liu HS, Zhu H, Cheng JD, Liu YL, Li AM, Zhang Y. Exposure to 1950-MHz TD-SCDMA electromagnetic fields affects the apoptosis of astrocytes via caspase-3-dependent pathway. PLoS One. 2012; 7:e42332. PMID: 22870319.
Article2. Gherardini L, Ciuti G, Tognarelli S, Cinti C. Searching for the perfect wave: the effect of radiofrequency electromagnetic fields on cells. Int J Mol Sci. 2014; 15:5366–5387. PMID: 24681584.
Article3. Tang J, Zhang Y, Yang L, Chen Q, Tan L, Zuo S, Feng H, Chen Z, Zhu G. Exposure to 900 MHz electromagnetic fields activates the mkp-1/ERK pathway and causes blood-brain barrier damage and cognitive impairment in rats. Brain Res. 2015; 1601:92–101. PMID: 25598203.4. Röösli M. Radiofrequency electromagnetic field exposure and non-specific symptoms of ill health: a systematic review. Environ Res. 2008; 107:277–287. PMID: 18359015.
Article5. Aldad TS, Gan G, Gao XB, Taylor HS. Fetal radiofrequency radiation exposure from 800-1900 mhz-rated cellular telephones affects neurodevelopment and behavior in mice. Sci Rep. 2012; 2:312. PMID: 22428084.
Article6. Hao D, Yang L, Chen S, Tong J, Tian Y, Su B, Wu S, Zeng Y. Effects of long-term electromagnetic field exposure on spatial learning and memory in rats. Neurol Sci. 2013; 34:157–164. PMID: 22362331.
Article7. Liu K, Zhang G, Wang Z, Liu Y, Dong J, Dong X, Liu J, Cao J, Ao L, Zhang S. The protective effect of autophagy on mouse spermatocyte derived cells exposure to 1800MHz radiofrequency electromagnetic radiation. Toxicol Lett. 2014; 228:216–224. PMID: 24813634.
Article8. Kim JH, Huh YH, Kim HR. Induction of Autophagy in the Striatum and Hypothalamus of Mice after 835 MHz Radiofrequency Exposure. PLoS One. 2016; 11:e0153308. PMID: 27073885.
Article9. Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004; 306:990–995. PMID: 15528435.
Article10. Shipp S. Structure and function of the cerebral cortex. Curr Biol. 2007; 17:R443–R449. PMID: 17580069.
Article11. Gray JR, Thompson PM. Neurobiology of intelligence: science and ethics. Nat Rev Neurosci. 2004; 5:471–482. PMID: 15152197.
Article12. Ballmaier M, O'Brien JT, Burton EJ, Thompson PM, Rex DE, Narr KL, McKeith IG, DeLuca H, Toga AW. Comparing gray matter loss profiles between dementia with Lewy bodies and Alzheimer's disease using cortical pattern matching: diagnosis and gender effects. Neuroimage. 2004; 23:325–335. PMID: 15325380.
Article13. Ortolano S, Vieitez I, Agis-Balboa RC, Spuch C1. Loss of GABAergic cortical neurons underlies the neuropathology of Lafora disease. Mol Brain. 2014; 7:7. PMID: 24472629.
Article14. Blakemore C, Jennett S. Brain stem. Oxford: The Oxford Companion to the Body;2001. cited 2016 Jul 8. Available from: http://www.encyclopedia.com/doc/1O128-brainstem.html.15. Althaus M, Van Roon AM, Mulder LJ, Mulder G, Aarnoudse CC, Minderaa RB. Autonomic response patterns observed during the performance of an attention-demanding task in two groups of children with autistic-type difficulties in social adjustment. Psychophysiology. 2004; 41:893–904. PMID: 15563342.
Article16. Kwon S, Kim J, Choe BH, Ko C, Park S. Electrophysiologic assessment of central auditory processing by auditory brainstem responses in children with autism spectrum disorders. J Korean Med Sci. 2007; 22:656–659. PMID: 17728505.
Article17. Fenik VB. Revisiting antagonist effects in hypoglossal nucleus: brainstem circuit for the state-dependent control of hypoglossal motoneurons: a hypothesis. Front Neurol. 2015; 6:254. PMID: 26648908.
Article18. Huber R, Treyer V, Schuderer J, Berthold T, Buck A, Kuster N, Landolt HP, Achermann P. Exposure to pulse-modulated radio frequency electromagnetic fields affects regional cerebral blood flow. Eur J Neurosci. 2005; 21:1000–1006. PMID: 15787706.
Article19. Xu S, Zhou Z, Zhang L, Yu Z, Zhang W, Wang Y, Wang X, Li M, Chen Y, Chen C, He M, Zhang G, Zhong M. Exposure to 1800 MHz radiofrequency radiation induces oxidative damage to mitochondrial DNA in primary cultured neurons. Brain Res. 2010; 1311:189–196. PMID: 19879861.20. Volkow ND, Tomasi D, Wang GJ, Vaska P, Fowler JS, Telang F, Alexoff D, Logan J, Wong C. Effects of cell phone radiofrequency signal exposure on brain glucose metabolism. JAMA. 2011; 305:808–813. PMID: 21343580.
Article21. Maskey D, Kim HG, Suh MW, Roh GS, Kim MJ. Alteration of glycine receptor immunoreactivity in the auditory brainstem of mice following three months of exposure to radiofrequency radiation at SAR 4.0 W/kg. Int J Mol Med. 2014; 34:409–419. PMID: 24866721.
Article22. Maskey D, Kim M, Aryal B, Pradhan J, Choi IY, Park KS, Son T, Hong SY, Kim SB, Kim HG, Kim MJ. Effect of 835 MHz radiofrequency radiation exposure on calcium binding proteins in the hippocampus of the mouse brain. Brain Res. 2010; 1313:232–241. PMID: 19968972.23. Yang C, Kaushal V, Shah SV, Kaushal GP. Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. Am J Physiol Renal Physiol. 2008; 294:F777–F787. PMID: 18256309.
Article24. Nassif M, Valenzuela V, Rojas-Rivera D, Vidal R, Matus S, Castillo K, Fuentealba Y, Kroemer G, Levine B, Hetz C. Pathogenic role of BECN1/Beclin 1 in the development of amyotrophic lateral sclerosis. Autophagy. 2014; 10:1256–1271. PMID: 24905722.
Article25. He C, Wei Y, Sun K, Li B, Dong X, Zou Z, Liu Y, Kinch LN, Khan S, Sinha S, Xavier RJ, Grishin NV, Xiao G, Eskelinen EL, Scherer PE, Whistler JL, Levine B. Beclin 2 functions in autophagy, degradation of G protein-coupled receptors, and metabolism. Cell. 2013; 154:1085–1099. PMID: 23954414.
Article26. Mariño G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014; 15:81–94. PMID: 24401948.
Article27. Chen Y, Klionsky DJ. The regulation of autophagy - unanswered questions. J Cell Sci. 2011; 124:161–170. PMID: 21187343.
Article28. Kabeya Y, Mizushima N, Yamamoto A, Oshitani-Okamoto S, Ohsumi Y, Yoshimori T. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J Cell Sci. 2004; 117:2805–2812. PMID: 15169837.
Article29. Maskey D, Yousefi S, Schmid I, Zlobec I, Perren A, Friis R, Simon HU. ATG5 is induced by DNA-damaging agents and promotes mitotic catastrophe independent of autophagy. Nat Commun. 2013; 4:2130. PMID: 23945651.
Article30. Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013; 19:983–997. PMID: 23921753.
Article31. Marchesi N, Osera C, Fassina L, Amadio M, Angeletti F, Morini M, Magenes G, Venturini L, Biggiogera M, Ricevuti G, Govoni S, Caorsi S, Pascale A, Comincini S. Autophagy is modulated in human neuroblastoma cells through direct exposition to low frequency electromagnetic fields. J Cell Physiol. 2014; 229:1776–1786. PMID: 24676932.
Article32. Zhu H, Wu H, Liu X, Li B, Chen Y, Ren X, Liu CG, Yang JM. Regulation of autophagy by a beclin 1-targeted microRNA, miR-30a, in cancer cells. Autophagy. 2009; 5:816–823. PMID: 19535919.
Article33. Köktürk S, Yardimoglu M, Celikozlu SD, Dolanbay EG, Cimbiz A. Effect of lycopersicon esculentum extract on apoptosis in the rat cerebellum, following prenatal and postnatal exposure to an electromagnetic field. Exp Ther Med. 2013; 6:52–56. PMID: 23935717.34. Salminen A, Kaarniranta K, Kauppinen A. Beclin 1 interactome controls the crosstalk between apoptosis, autophagy and inflammasome activation: impact on the aging process. Ageing Res Rev. 2013; 12:520–534. PMID: 23220384.
Article35. Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005; 122:927–939. PMID: 16179260.
Article36. Sendrowski K, Rusak M, Sobaniec P, Iłendo E, Dąbrowska M, Boćkowski L, Koput A, Sobaniec W. Study of the protective effect of calcium channel blockers against neuronal damage induced by glutamate in cultured hippocampal neurons. Pharmacol Rep. 2013; 65:730–736. PMID: 23950597.
Article37. Sun ZC, Ge JL, Guo B, Guo J, Hao M, Wu YC, Lin YA, La T, Yao PT, Mei YA, Feng Y, Xue L. Extremely low frequency electromagnetic fields facilitate vesicle endocytosis by increasing presynaptic calcium channel expression at a central synapse. Sci Rep. 2016; 6:21774. PMID: 26887777.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Exposure to 835 MHz RF-EMF decreases the expression of calcium channels, inhibits apoptosis, but induces autophagy in the mouse hippocampus
- The Effect of 835 MHz Radiofrequency Radiation Exposure on the Immunohistochemical Distribution of Calbindin D28k and Calretinin in the Mouse Cerebellar Cortex
- Effects of radiofrequency exposure emitted from a GSM mobile phone on proliferation, differentiation, and apoptosis of neural stem cells
- Possible Effects of Radiofrequency Electromagnetic Field Exposure on Central Nerve System
- Prediction of the Exposure to 1763MHz Radiofrequency Radiation Based on Gene Expression Patterns