Ann Lab Med.
2015 Sep;35(5):510-518. 10.3343/alm.2015.35.5.510.
Routine Chromosomal Microarray Analysis is Necessary in Korean Patients With Unexplained Developmental Delay/Mental Retardation/Autism Spectrum Disorder
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea. KAL1119@yuhs.ac
- 2Department of Laboratory Medicine, Kosin University College of Medicine, Busan, Korea.
- KMID: 2369767
- DOI: http://doi.org/10.3343/alm.2015.35.5.510
Abstract
- BACKGROUND
All over the world, chromosomal microarray (CMA) is now the first tier diagnostic assay for genetic testing to evaluate developmental delay (DD), mental retardation (MR), and autism spectrum disorder (ASD) with unknown etiology. The average diagnostic yield of the CMA test is known to be about 12.2%, while that of conventional G-banding karyotype is below 3%. This study aimed to assess the usefulness of CMA for the purpose of clinical diagnostic testing in the Korean population.
METHODS
We performed CMA and multiplex ligation-dependent probe amplification (MLPA) tests in 96 patients with normal karyotype and unexplained DD, MR, or ASD. The CMA was conducted with CytoScan 750K array (Affymetrix, USA) with an average resolution of 100 kb.
RESULTS
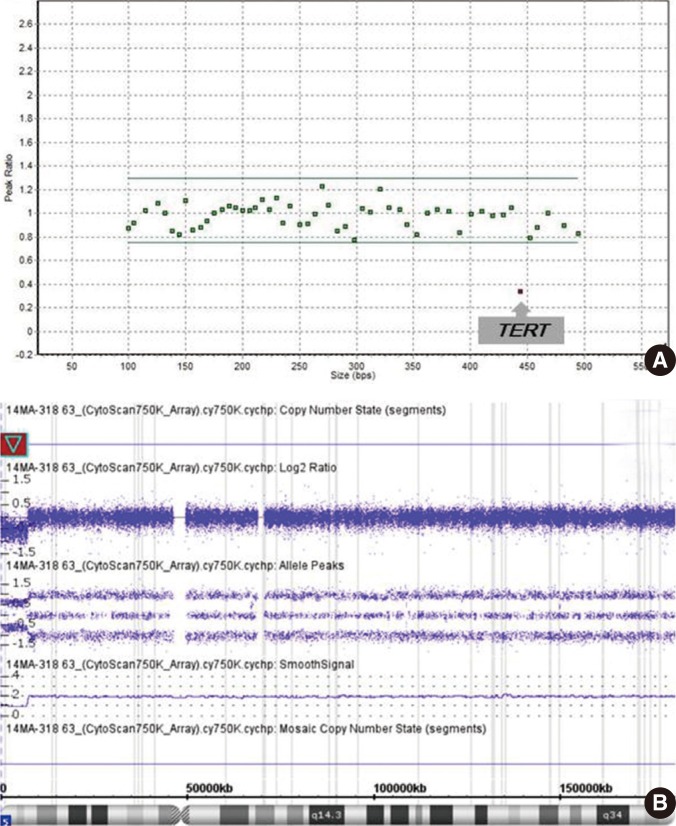
Pathogenic copy number variations (CNVs) were detected in 15 patients by CMA and in two patients by MLPA for four known microdeletion syndromes (Prader-Willi/Angelman syndrome, DiGeorge syndrome, Miller-Dieker syndrome and Williams syndrome) designated by National Health Insurance system in Korea. The diagnostic yield was 15.6% and 2.1%, respectively. Thirteen (13.5%) patients (excluding cases with pathogenic CNVs) had variants of uncertain clinical significance. There was one patient with a 17.1-megabase (Mb) region of homozygosity on chromosome 4q.
CONCLUSIONS
Our findings suggest the necessity of CMA as a routine diagnostic test for unexplained DD, MR, and ASD in Korea.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Clinical Utility of Methylation-Specific Multiplex Ligation-Dependent Probe Amplification for the Diagnosis of Prader–Willi Syndrome and Angelman Syndrome
Boram Kim, Yongsook Park, Sung Im Cho, Man Jin Kim, Jong-Hee Chae, Ji Yeon Kim, Moon-Woo Seong, Sung Sup Park
Ann Lab Med. 2022;42(1):79-88. doi: 10.3343/alm.2022.42.1.79.
Reference
-
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, D.C.: American Psychiatric Association;2013.2. Battaglia A, Doccini V, Bernardini L, Novelli A, Loddo S, Capalbo A, et al. Confirmation of chromosomal microarray as a first-tier clinical diagnostic test for individuals with developmental delay, intellectual disability, autism spectrum disorders and dysmorphic features. Eur J Paediatr Neurol. 2013; 17:589–599. PMID: 23711909.
Article3. Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008; 358:667–675. PMID: 18184952.
Article4. Moeschler JB, Shevell M. American Academy of Pediatrics Committee on Genetics. Clinical genetic evaluation of the child with mental retardation or developmental delays. Pediatrics. 2006; 117:2304–2316. PMID: 16740881.
Article5. Miller DT, Adam MP, Aradhya S, Biesecker LG, Brothman AR, Carter NP, et al. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet. 2010; 86:749–764. PMID: 20466091.
Article6. Rauch A, Hoyer J, Guth S, Zweier C, Kraus C, Becker C, et al. Diagnostic yield of various genetic approaches in patients with unexplained developmental delay or mental retardation. Am J Med Genet A. 2006; 140:2063–2074. PMID: 16917849.
Article7. Wagenstaller J, Spranger S, Lorenz-Depiereux B, Kazmierczak B, Nathrath M, Wahl D, et al. Copy-number variations measured by single-nucleotide-polymorphism oligonucleotide arrays in patients with mental retardation. Am J Hum Genet. 2007; 81:768–779. PMID: 17847001.
Article8. Bi W, Borgan C, Pursley AN, Hixson P, Shaw CA, Bacino CA, et al. Comparison of chromosome analysis and chromosomal microarray analysis: what is the value of chromosome analysis in today's genomic array era? Genet Med. 2013; 15:450–457. PMID: 23238528.
Article9. Kearney HM, Thorland EC, Brown KK, Quintero-Rivera F, South ST. American College of Medical Genetics standards and guidelines for interpretation and reporting of postnatal constitutional copy number variants. Genet Med. 2011; 13:680–685. PMID: 21681106.
Article10. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17:405–423. PMID: 25741868.
Article11. Xiang B, Zhu H, Shen Y, Miller DT, Lu K, Hu X, et al. Genome-wide oligonucleotide array comparative genomic hybridization for etiological diagnosis of mental retardation: a multicenter experience of 1499 clinical cases. J Mol Diagn. 2010; 12:204–212. PMID: 20093387.12. Pinto D, Marshall C, Feuk L, Scherer SW. Copy-number variation in control population cohorts. Hum Mol Genet. 2007; 16(Spec No. 2):R168–R173. PMID: 17911159.
Article13. Glancy M, Barnicoat A, Vijeratnam R, de Souza S, Gilmore J, Huang S, et al. Transmitted duplication of 8p23.1-8p23.2 associated with speech delay, autism and learning difficulties. Eur J Hum Genet. 2009; 17:37–43. PMID: 18716609.
Article14. Parisi MA, Bennett CL, Eckert ML, Dobyns WB, Gleeson JG, Shaw DW, et al. The NPHP1 gene deletion associated with juvenile nephronophthisis is present in a subset of individuals with Joubert syndrome. Am J Hum Genet. 2004; 75:82–91. PMID: 15138899.
Article15. Adly N, Alhashem A, Ammari A, Alkuraya FS. Ciliary genes TBC1D32/C6orf170 and SCLT1 are mutated in patients with OFD type IX. Hum Mutat. 2014; 35:36–40. PMID: 24285566.16. Nowaczyk MJ, Irons MB. Smith-Lemli-Opitz syndrome: phenotype, natural history, and epidemiology. Am J Med Genet C Semin Med Genet. 2012; 160C:250–262. PMID: 23059950.
Article17. Weaver DD, Solomon BD, Akin-Samson K, Kelley RI, Muenke M. Cyclopia (synophthalmia) in Smith-Lemli-Opitz syndrome: first reported case and consideration of mechanism. Am J Med Genet C Semin Med Genet. 2010; 154C:142–145. PMID: 20104611.
Article18. Vona B, Nanda I, Neuner C, Schröder J, Kalscheuer VM, Shehata-Dieler W, et al. Terminal chromosome 4q deletion syndrome in an infant with hearing impairment and moderate syndromic features: review of literature. BMC Med Genet. 2014; 15:72. PMID: 24962056.
Article19. Bernardini L, Sinibaldi L, Capalbo A, Bottillo I, Mancuso B, Torres B, et al. HDR (Hypoparathyroidism, Deafness, Renal dysplasia) syndrome associated to GATA3 gene duplication. Clin Genet. 2009; 76:117–119. PMID: 19659764.20. Cingoz S, Bisgaard AM, Bache I, Bryndorf T, Kirchoff M, Petersen W, et al. 4q35 deletion and 10p15 duplication associated with immunodeficiency. Am J Med Genet A. 2006; 140:2231–2235. PMID: 16964622.
Article21. Papenhausen P, Schwartz S, Risheg H, Keitges E, Gadi I, Burnside RD, et al. UPD detection using homozygosity profiling with a SNP genotyping microarray. Am J Med Genet A. 2011; 155A:757–768. PMID: 21594998.
Article22. Rehder CW, David KL, Hirsch B, Toriello HV, Wilson CM, Kearney HM. American College of Medical Genetics and Genomics: standards and guidelines for documenting suspected consanguinity as an incidental finding of genomic testing. Genet Med. 2013; 15:150–152. PMID: 23328890.
Article23. Middleton FA, Trauzzi MG, Shrimpton AE, Gentile KL, Morley CP, Medeiros H, et al. Complete maternal uniparental isodisomy of chromosome 4 in a subject with major depressive disorder detected by high density SNP genotyping arrays. Am J Med Genet B Neuropsychiatr Genet. 2006; 141B:28–32. PMID: 16331669.
Article24. Ramanathan S, Woodroffe A, Flodman PL, Mays LZ, Hanouni M, Modahl CB, et al. A case of autism with an interstitial deletion on 4q leading to hemizygosity for genes encoding for glutamine and glycine neurotransmitter receptor sub-units (AMPA 2, GLRA3, GLRB) and neuropeptide receptors NPY1R, NPY5R. BMC Med Genet. 2004; 5:10. PMID: 15090072.
Article25. Taylor CF, Charlton RS, Burn J, Sheridan E, Taylor GR. Genomic deletions in MSH2 or MLH1 are a frequent cause of hereditary non-polyposis colorectal cancer: identification of novel and recurrent deletions by MLPA. Hum Mutat. 2003; 22:428–433. PMID: 14635101.26. Zhang X, Snijders A, Segraves R, Zhang X, Niebuhr A, Albertson D, et al. High-resolution mapping of genotype-phenotype relationships in cri du chat syndrome using array comparative genomic hybridization. Am J Hum Genet. 2005; 76:312–326. PMID: 15635506.
Article27. Wordsworth S, Buchanan J, Regan R, Davison V, Smith K, Dyer S, et al. Diagnosing idiopathic learning disability: a cost-effectiveness analysis of microarray technology in the National Health Service of the United Kingdom. Genomic Med. 2007; 1:35–45. PMID: 18923927.
Article28. Trakadis Y, Shevell M. Microarray as a first genetic test in global developmental delay: a cost-effectiveness analysis. Dev Med Child Neurol. 2011; 53:994–999. PMID: 21848878.
Article29. Coulter ME, Miller DT, Harris DJ, Hawley P, Picker J, Roberts AE, et al. Chromosomal microarray testing influences medical management. Genet Med. 2011; 13:770–776. PMID: 21716121.
Article30. Roberts JL, Hovanes K, Dasouki M, Manzardo AM, Butler MG. Chromosomal microarray analysis of consecutive individuals with autism spectrum disorders or learning disability presenting for genetic services. Gene. 2014; 535:70–78. PMID: 24188901.
Article31. Nicholl J, Waters W, Mulley JC, Suwalski S, Brown S, Hull Y, et al. Cognitive deficit and autism spectrum disorders: prospective diagnosis by array CGH. Pathology. 2014; 46:41–45. PMID: 24300712.
Article32. Bonaglia MC, Giorda R, Beri S, De Agostini C, Novara F, Fichera M, et al. Molecular mechanisms generating and stabilizing terminal 22q13 deletions in 44 subjects with Phelan/McDermid syndrome. PLoS Genet. 2011; 7:e1002173. PMID: 21779178.
Article33. Bonaglia MC, Giorda R, Borgatti R, Felisari G, Gagliardi C, Selicorni A, et al. Disruption of the ProSAP2 gene in a t(12;22)(q24.1;q13.3) is associated with the 22q13.3 deletion syndrome. Am J Hum Genet. 2001; 69:261–268. PMID: 11431708.
Article34. Gong X, Jiang YW, Zhang X, An Y, Zhang J, Wu Y, et al. High proportion of 22q13 deletions and SHANK3 mutations in Chinese patients with intellectual disability. PLoS One. 2012; 7:e34739. PMID: 22509352.
Article35. Lee KA. The genetic testing system-Pending issues and future extensions. Medical Review. 2014; 11:22–28. http://mdzone.co.kr/news/view.php?idx=13923 (Updated on April 2014).36. Thevenon J, Callier P, Poquet H, Bache I, Menten B, Malan V, et al. 3q27.3 microdeletional syndrome: a recognisable clinical entity associating dysmorphic features, marfanoid habitus, intellectual disability and psychosis with mood disorder. J Med Genet. 2014; 51:21–27. PMID: 24133203.
Article37. Pehlivan T, Pober BR, Brueckner M, Garrett S, Slaugh R, Van Rheeden R, et al. GATA4 haploinsufficiency in patients with interstitial deletion of chromosome region 8p23.1 and congenital heart disease. Am J Med Genet. 1999; 83:201–206. PMID: 10096597.
Article38. Ballarati L, Cereda A, Caselli R, Selicorni A, Recalcati MP, Maitz S, et al. Genotype-phenotype correlations in a new case of 8p23.1 deletion and review of the literature. Eur J Med Genet. 2011; 54:55–59. PMID: 20969981.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chromosomal Microarray Testing in 42 Korean Patients with Unexplained Developmental Delay, Intellectual Disability, Autism Spectrum Disorders, and Multiple Congenital Anomalies
- Diagnostic distal 16p11.2 deletion in a preterm infant with facial dysmorphism
- Recent update of autism spectrum disorders
- Clinical Applications of Chromosomal Microarray Analysis
- The Findings of 1H Magnetic Resonance Spectroscopy in Children with Mental Retardation or Autism