Ann Lab Med.
2017 May;37(3):254-260. 10.3343/alm.2017.37.3.254.
Effects of Neutralization by Soluble ABH Antigens Produced by Transplanted Kidneys From ABO-Incompatible Secretor Donors
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea. hyunok1019@yuhs.ac
- 2Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Surgery, Yonsei University College of Medicine, Seoul, Korea. ysms91@yuhs.ac
- 4Research Institute for Transplantation, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2369751
- DOI: http://doi.org/10.3343/alm.2017.37.3.254
Abstract
- BACKGROUND
Grafts survive despite blood group antigens on the transplant being continuously exposed to antibodies in the blood of recipients in ABO-incompatible kidney transplantation (ABOi KT), owing to the mechanism of accommodation. We analyzed the immunodynamics of soluble ABH antigens in allografts from secretor donors and the influence of such immunodynamics on accommodation and subsequent graft survival in ABOi KT.
METHODS
The genotype of a known human β-galactoside α-1,2-fucosyltransferase gene (FUT2), which determines soluble ABH antigen secretor status, was established in 32 donors for ABOi KT at the Severance Hospital, from June 2010 to July 2015. Clinical outcomes of recipients, such as anti-A/B antibody titer change, renal function, and graft survival, were evaluated.
RESULTS
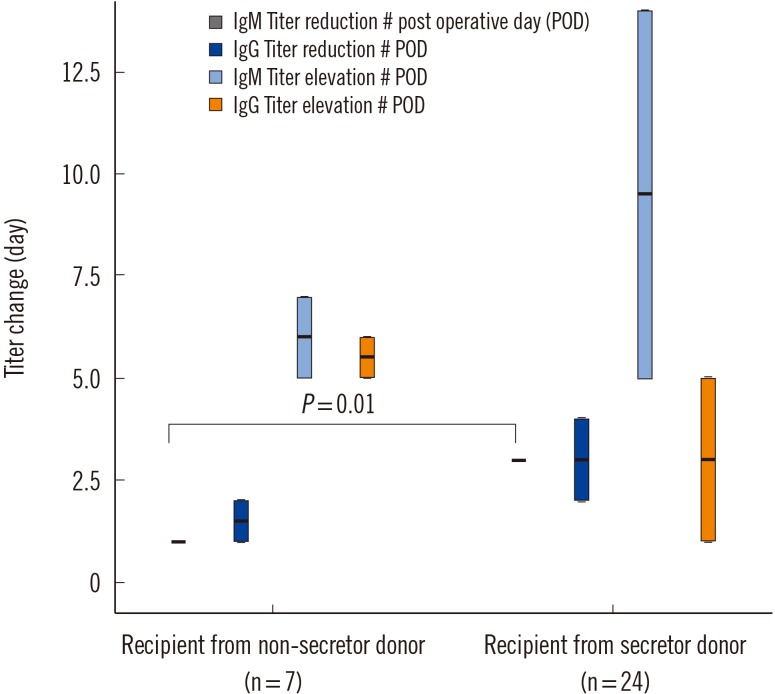
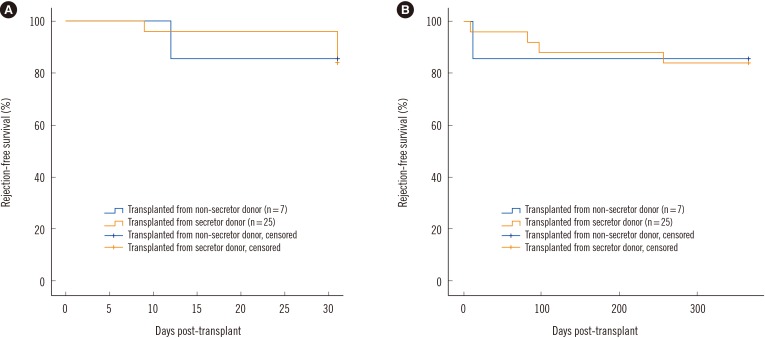
Twenty-five donors were secretors (78.1%), and seven were nonsecretors (21.9%). The frequency of anti-A/B IgG or IgM antibody titer elevation or reduction post-transplantation was not significantly related to donor secretor status. However, IgM titer was rapidly reduced in recipients transplanted from nonsecretor donors (P=0.01), which could be explained by the lack of absorption effect of soluble antigens, enhancing the binding of antibodies to antigens in the allografts. Interestingly, soluble ABH antigens did not affect rejection-free graft survival, which may be due to the nature of β-galactoside α-1,2-fucosyltransferase.
CONCLUSIONS
Soluble ABH antigens produced by transplanted kidneys from secretor donors played a role in inducing accommodation within three months of KT through neutralization; however, major graft outcomes were not affected.
Keyword
MeSH Terms
Figure
Reference
-
1. Nelson PW, Shield CF 3rd, Muruve NA, Murillo D, Warady BA, Aeder MI, et al. Increased access to transplantation for blood group B cadaveric waiting list candidates by using A2 kidneys: time for a new national system? Am J Transplant. 2002; 2:94–99. PMID: 12095063.2. Porter KA. Morphological aspects of renal homograft rejection. Br Med Bull. 1965; 21:171–175. PMID: 14313592.3. Yoo S, Lee EY, Huh KH, Kim MS, Kim YS, Kim HO. Role of plasma exchange in ABO-incompatible kidney transplantation. Ann Lab Med. 2012; 32:283–288. PMID: 22779070.4. Cosio FG, Lager DJ, Lorenz EC, Amer H, Gloor JM, Stegall MD. Significance and implications of capillaritis during acute rejection of kidney allografts. Transplantation. 2010; 89:1088–1094. PMID: 20216485.5. Tantravahi J, Womer KL, Kaplan B. Why hasn't eliminating acute rejection improved graft survival? Annu Rev Med. 2007; 58:369–385. PMID: 17002551.6. Ravn V, Dabelsteen E. Tissue distribution of histo-blood group antigens. APMIS. 2000; 108:1–28. PMID: 10698081.7. Oriol R, Cartron JP, Cartron J, Mulet C. Biosynthesis of ABH and Lewis antigens in normal and transplanted kidneys. Transplantation. 1980; 29:184–188. PMID: 6987782.8. Ulfvin A, Bäcker AE, Clausen H, Hakomori S, Rydberg L, Samuelsson BE, et al. Expression of glycolipid blood group antigens in single human kidneys: change in antigen expression of rejected ABO incompatible kidney grafts. Kidney Int. 1993; 44:1289–1297. PMID: 7508004.9. Kumazaki T, Yoshida A. Biochemical evidence that secretor gene, Se, is a structural gene encoding a specific fucosyltransferase. Proc Natl Acad Sci USA. 1984; 81:4193–4197. PMID: 6588382.10. Sarnesto A, Köhlin T, Hindsgaul O, Vogele K, Blaszczyk-Thurin M, Thurin J. Purification of the β-N-acetylglucosaminide α 1→3-fucosyltransferase from human serum. J Biol Chem. 1992; 267:2745–2752. PMID: 1733970.11. Wilbrandt R, Tung KS, Deodhar SD, Nakamoto S, Kolff WJ. ABO blood group incompatibility in human renal homotransplantation. Am J Clin Pathol. 1969; 51:15–23. PMID: 4885605.12. Montgomery RA, Hardy MA, Jordan SC, Racusen LC, Ratner LE, Tyan DB, et al. Consensus opinion from the antibody working group on the diagnosis, reporting, and risk assessment for antibody-mediated rejection and desensitization protocols. Transplantation. 2004; 78:181–185. PMID: 15280674.13. Park WD, Grande JP, Ninova D, Nath KA, Platt JL, Gloor JM, et al. Accommodation in ABO-incompatible kidney allografts, a novel mechanism of self-protection against antibody-mediated injury. Am J Transplant. 2003; 3:952–960. PMID: 12859529.14. Takahashi K. Accommodation in ABO-incompatible kidney transplantation: why do kidney grafts survive? Transplant Proc. 2004; 36(2 Suppl):193S–196S. PMID: 15041335.15. Tang AH, Platt JL. Accommodation of grafts: implications for health and disease. Hum Immunol. 2007; 68:645–651. PMID: 17678718.16. Park KU, Song J, Han KS, Kim JQ. The fusion allele of the FUT2 (secretor type α(1,2)-fucosyltransferase) gene at a high frequency and a new se385 allele in a Korean population. Ann Hematol. 2005; 84:656–660. PMID: 15809881.17. Fung MK, Grossman BJ, editors. Technical manual. 18th ed. Bethesda: American Association of Blood Banks;2014.18. Mengel M, Sis B, Haas M, Colvin RB, Halloran PF, Racusen LC, et al. Banff 2011 Meeting report: new concepts in antibody-mediated rejection. Am J Transplant. 2012; 12:563–570. PMID: 22300494.19. Lakkis FG, Arakelov A, Konieczny BT, Inoue Y. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med. 2000; 6:686–688. PMID: 10835686.20. Lynch RJ, Platt JL. Accommodation in renal transplantation: unanswered questions. Curr Opin Organ Transplant. 2010; 15:481–485. PMID: 20613524.21. Platt JL, Vercellotti GM, Dalmasso AP, Matas AJ, Bolman RM, Najarian JS, et al. Transplantation of discordant xenografts: a review of progress. Immunol Today. 1990; 11:450–456. PMID: 2073317.22. Rydberg L. ABO-incompatibility in solid organ transplantation. Transfus Med. 2001; 11:325–342. PMID: 11532188.23. Costa C, Zhao L, Burton WV, Rasas C, Bondiol KR, Williams BL, et al. Transgenic pigs designed to express human CD59 and H-transferase to avoid humoral xenograft rejection. Xenotransplantation. 2002; 9:45–57. PMID: 12005104.24. Tabata T, de Perrot M, Keshavjee S, Liu M, Downey GP, Waddell TK. Accommodation after lung xenografting from hamster to rat. Transplantation. 2003; 75:607–612. PMID: 12640297.25. Kudo T, Iwasaki H, Nishihara S, Shinya N, Ando T, Narimatsu I, et al. Molecular genetic analysis of the human Lewis histo-blood group system. II. Secretor gene inactivation by a novel single missense mutation A385T in Japanese nonsecretor individuals. J Biol Chem. 1996; 271:9830–9837. PMID: 8621666.26. Moon CK, Kany YB, Lee DW, Kang DY, Lee D. Lewis phenotypes and ABH secretor status of Korean adults. Korean J Clin Pathol. 1984; 4:237–244.27. Suh IB, Hwang MW, Lim CS, Lee CK, Ma KR, Kim YK, et al. The genotyping of the secretory gene (Sec2) in the Korean population. Korean J Clin Pathol. 1999; 19:572–577.28. Stussi G, Huggel K, Lutz HU, Schanz U, Rieben R, Seebach JD. Isotype-specific detection of ABO blood group antibodies using a novel flow cytometric method. Br J Haematol. 2005; 130:954–963. PMID: 16156865.29. Halloran PF, Reeve JP, Pereira AB, Hidalgo LG, Famulski KS. Antibody-mediated rejection, T cell-mediated rejection, and the injury-repair response: new insights from the Genome Canada studies of kidney transplant biopsies. Kidney Int. 2014; 85:258–264. PMID: 23965521.30. Yoshida A, Schmidt GM, Blume KG, Beutler E. Plasma blood group glycosyltransferase activities after bone marrow transplantation. Blood. 1980; 55:699–701. PMID: 6766757.31. Takahashi K. A new concept of accommodation in ABO-incompatible kidney transplantation. Clin Transplant. 2005; 19(Suppl 14):76–85. PMID: 15955174.32. Won DI, Jung OJ, Lee YS, Kim SG, Suh JS. Flow cytometry antibody screening using pooled red cells. Cytometry B Clin Cytom. 2010; 78:96–104. PMID: 19714726.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ABO-Incompatible Kidney Transplantation
- Analysis of the Results of ABO-Incompatible Kidney Transplantation: In Comparison with ABO-Compatible Kidney Transplantation
- ABO-Incompatible Living Donor Liver Transplantation
- Clinical Outcomes between Living Related and Living Unrelated Kidney Transplantation in ABO-Incompatible Kidney Transplant Recipients
- The analysis of Secretory Gene (Fucosyltransferase II): The relationship between the genotype of the Secretory Gene (Fucosyltransferase II) and the secretory phenotype of the saliva