Ann Dermatol.
2014 Jun;26(3):357-362.
Investigation of the Degradation-Retarding Effect Caused by the Low Swelling Capacity of a Novel Hyaluronic Acid Filler Developed by Solid-Phase Crosslinking Technology
- Affiliations
-
- 1Amorepacific R&D Center, Yongin, Korea. beomjoon@unitel.co.kr
- 2Department of Dermatology, Chung-Ang University College of Medicine, Seoul, Korea.
Abstract
- BACKGROUND
A variety of hyaluronic acid (HA) fillers demonstrate unique physical characteristics, which affect the quality of the HA filler products. The critical factors that affect the degradation of HA gels have not yet been determined.
OBJECTIVE
Our objective was to determine the characteristics of HA gels that affect their resistance to the degradation caused by radicals and enzymes.
METHODS
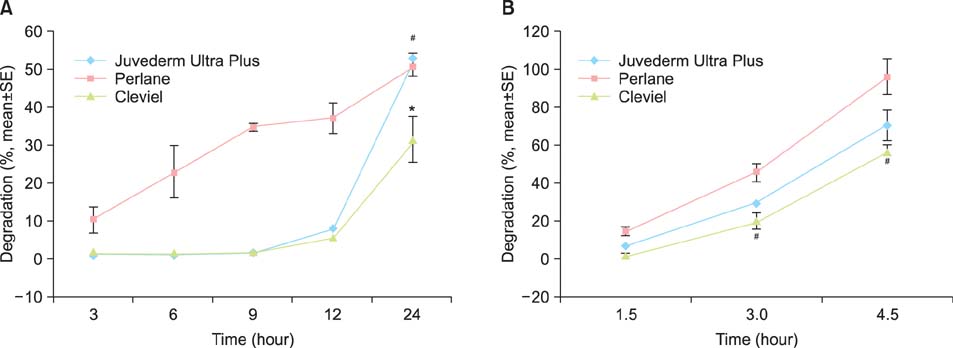
Three types of HA fillers for repairing deep wrinkles, Juvederm Ultra Plus (J-U), Restylane Perlane (Perlane), and Cleviel, were tested in this study. The resistance of these HA fillers to enzymatic degradation was measured by carbazole and displacement assays using hyaluronidase as the enzyme. The resistance of these fillers to radical degradation was measured by the displacement assay using H2O2.
RESULTS
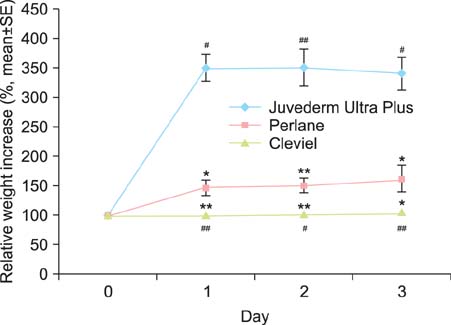
Different tests for evaluating the degradation resistance of HA gels can yield different results. The filler most susceptible to enzymatic degradation was J-U, followed by Perlane and Cleviel. The HA filler showing the highest degree of degradation caused by H2O2 treatment was Perlane, followed by J-U, and then Cleviel. Cleviel showed higher enzymatic and radical resistances than J-U and Perlane did. Furthermore, it exhibited the highest resistance to heat and the lowest swelling ratio among all the fillers that were examined.
CONCLUSION
The main factor determining the degradation of HA particles is the gel swelling ratio, which is related to the particle structure of the gel. Our in vitro assays suggest that the decrease in the swelling ratio will lead to a retarding effect on the degradation of HA fillers.
Keyword
MeSH Terms
Figure
Reference
-
1. Buck DW 2nd, Alam M, Kim JY. Injectable fillers for facial rejuvenation: a review. J Plast Reconstr Aesthet Surg. 2009; 62:11–18.
Article2. Beasley KL, Weiss MA, Weiss RA. Hyaluronic acid fillers: a comprehensive review. Facial Plast Surg. 2009; 25:86–94.
Article3. Volpi N, Schiller J, Stern R, Soltés L. Role, metabolism, chemical modifications and applications of hyaluronan. Curr Med Chem. 2009; 16:1718–1745.
Article4. Hahn SK, Park JK, Tomimatsu T, Shimoboji T. Synthesis and degradation test of hyaluronic acid hydrogels. Int J Biol Macromol. 2007; 40:374–380.
Article5. Ibrahim S, Kang QK, Ramamurthi A. The impact of hyaluronic acid oligomer content on physical, mechanical, and biologic properties of divinyl sulfone-crosslinked hyaluronic acid hydrogels. J Biomed Mater Res A. 2010; 94:355–370.
Article6. Bogdan Allemann I, Baumann L. Hyaluronic acid gel (Juvéderm) preparations in the treatment of facial wrinkles and folds. Clin Interv Aging. 2008; 3:629–634.7. Jones D, Tezel A, Borrell M. In vitro resistance to degradation of hyaluronic acid dermal fillers by ovine testicular hyaluronidase. Dermatol Surg. 2010; 36:804–809.
Article8. Sall I, Férard G. Comparison of the sensitivity of 11 cross-linked hyaluronic acid gels to bovine testis hyaluronidase. Polym Degrad Stab. 2007; 92:915–919.
Article9. Kablik J, Monheit GD, Yu L, Chang G, Gershkovich J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009; 35:Suppl 1. 302–312.
Article10. Bitter T, Muir HM. A modified uronic acid carbazole reaction. Anal Biochem. 1962; 4:330–334.
Article11. Vercruysse KP, Marecak DM, Marecek JF, Prestwich GD. Synthesis and in vitro degradation of new polyvalent hydrazide cross-linked hydrogels of hyaluronic acid. Bioconjug Chem. 1997; 8:686–694.
Article12. Lee A, Grummer SE, Kriegel D, Marmur E. Hyaluronidase. Dermatol Surg. 2010; 36:1071–1077.
Article13. Burdick JA, Chung C, Jia X, Randolph MA, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005; 6:386–391.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hyaluronidase: An overview of its properties, applications, and side effects
- Facial Pseudocyst Caused by Hyaluronic Acid Filler Injection: A Case Report
- Treatment of Lower Eyelid Swelling after Retrobulbar Hyaluronic Acid Filler Injection in Phthisis Bulbi
- Overview of Filler: Compositions, Effects, Rheological Consideration
- A Case of Rhabdomyosarcoma Mimicking Complications of Hyaluronic Acid Filler Injection