Investig Clin Urol.
2017 Jan;58(1):61-69. 10.4111/icu.2017.58.1.61.
Prospective study analyzing risk factors and characteristics of healthcare-associated infections in a Urology ward
- Affiliations
-
- 1Department of Urology, Hospital Universitario 12 de Octubre, Madrid, Spain. josemedinapolo@movistar.es
- KMID: 2365098
- DOI: http://doi.org/10.4111/icu.2017.58.1.61
Abstract
- PURPOSE
Healthcare-associated infections (HAIs) in urological patients have special features due to specific risk factors. Our objective was to evaluate the characteristics and risk factors for HAIs in patients hospitalized in a Urology ward.
MATERIALS AND METHODS
We evaluated prospectively, from 2012 to 2015, the incidence, types and risk factor for HAIs, microbiological and resistance patterns.
RESULTS
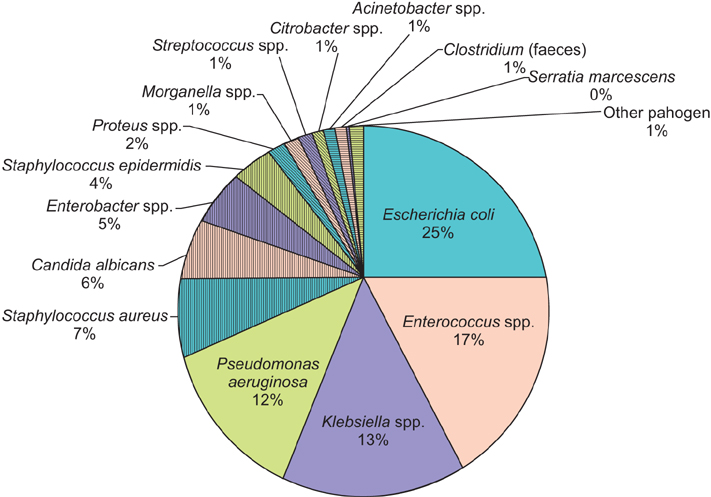
The incidence of HAIs was 6.3%. The most common types were urinary infections (70.5%) and surgical site infections (22.1%). Univariate analysis showed an increased risk of HAIs among patients with American Society of Anesthesiologists physical status classification system III-IV (odds ratio [OR], 1.39; p<0.001), immunosuppression (OR, 1.80; p=0.013), previous urinary infection (OR, 4.46; p<0,001), and urinary catheter before admission (OR, 1.74; p<0.001). The surgical procedures with the highest incidence of HAIs were radical cystectomy (54.2%) and renal surgery (8.7%). The most frequently isolated microorganisms were Escherichia coli (25.1%), Enterococcus spp. (17.5%), Klebsiella spp. (13.5%) and Pseudomonas aeruginosa (12.3%). Enterococcus sp was the most common microorganism after radical cystectomy and in surgical site infections, E. coli showed resistance rates of 53.5% for fluoroquinolones, 9.3% for amikacin. The percentage of extended-spectrum betalactamase producing E. coli was 24.7%. Klebsiella spp. showed resistance rates of 47.8% for fluoroquinolones, 7.1% for amikacin and 4.3% for carbapenems. Enterococcus spp showed resistance rates of 1.7% for vancomycin and; P. aeruginosa of 33.3% for carbapenems and 26.2% for amikacin.
CONCLUSIONS
Comorbidities, previous urinary infections, and urinary catheter are risk factors for HAIs. The microorganisms most commonly isolated were E. coli, Enterococcus and P. aeruginosa. Prospective monitoring may decrease the incidence of infections.
Keyword
MeSH Terms
-
Amikacin
Carbapenems
Classification
Comorbidity
Cystectomy
Drug Resistance, Multiple
Enterococcus
Escherichia coli
Fluoroquinolones
Humans
Immunosuppression
Incidence
Infection Control
Klebsiella
Prospective Studies*
Pseudomonas aeruginosa
Risk Factors*
Surgical Wound Infection
Urinary Catheters
Urinary Tract Infections
Urology Department, Hospital
Urology*
Vancomycin
Amikacin
Carbapenems
Fluoroquinolones
Vancomycin
Figure
Reference
-
1. Horcajada JP, Shaw E, Padilla B, Pintado V, Calbo E, Benito N, et al. Healthcare-associated, community-acquired and hospital-acquired bacteraemic urinary tract infections in hospitalized patients: a prospective multicentre cohort study in the era of antimicrobial resistance. Clin Microbiol Infect. 2013; 19:962–968.2. Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, et al. Health care--associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002; 137:791–797.3. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008; 36:309–332.4. Lee SY, Kotapati S, Kuti JL, Nightingale CH, Nicolau DP. Impact of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species on clinical outcomes and hospital costs: a matched cohort study. Infect Control Hosp Epidemiol. 2006; 27:1226–1232.5. Johansen TE. Nosocomially acquired urinary tract infections in urology departments. Why an international prevalence study is needed in urology. Int J Antimicrob Agents. 2004; 23:Suppl 1. S30–S34.6. Centers for Disease Control and Prevention. Healthcare-associated infections [Internet]. Atlanta (GA): Centers for Disease Control and Prevention;2016. cited 2016 Nov 9. Available from: https://www.cdc.gov/hai/.7. Aguilar-Duran S, Horcajada JP, Sorlí L, Montero M, Salvadó M, Grau S, et al. Community-onset healthcare-related urinary tract infections: comparison with community and hospital-acquired urinary tract infections. J Infect. 2012; 64:478–483.8. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999; 27:97–132.9. Clinical and Laboratory Standards Institute. CLSI Standards and Guidelines - CLSI Standards Center [Internet]. Wayne(PA): Clinical and Laboratory Standards Institute;2016. cited 2016 Nov 17. Available from: http://clsi.org/standards/.10. Bjerklund Johansen TE, Cek M, Naber K, Stratchounski L, Svendsen MV, Tenke P, et al. Prevalence of hospital-acquired urinary tract infections in urology departments. Eur Urol. 2007; 51:1100–1111.11. Cek M, Tandoğdu Z, Wagenlehner F, Tenke P, Naber K, Bjerklund-Johansen TE. Healthcare-associated urinary tract infections in hospitalized urological patients: a global perspective: results from the GPIU studies 2003-2010. World J Urol. 2014; 32:1587–1594.12. Cullen IM, Manecksha RP, McCullagh E, Ahmad S, O'Kelly F, Flynn R, et al. An 11-year analysis of the prevalent uropathogens and the changing pattern of Escherichia coli antibiotic resistance in 38,530 community urinary tract infections, Dublin 1999-2009. Ir J Med Sci. 2013; 182:81–89.13. Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010; 50:625–663.14. Di Pietrantonj C, Ferrara L, Lomolino G. Multicenter study of the prevalence of nosocomial infections in Italian hospitals. Infect Control Hosp Epidemiol. 2004; 25:85–87.15. Smyth ET, McIlvenny G, Enstone JE, Emmerson AM, Humphreys H, Fitzpatrick F, et al. Four country healthcare associated infection prevalence survey 2006: overview of the results. J Hosp Infect. 2008; 69:230–248.16. Wagenlehner FM, Cek M, Naber KG, Kiyota H, Bjerklund-Johansen TE. Epidemiology, treatment and prevention of healthcare-associated urinary tract infections. World J Urol. 2012; 30:59–67.17. Matsumoto T, Kiyota H, Matsukawa M, Yasuda M, Arakawa S, Monden K, et al. Japanese guidelines for prevention of perioperative infections in urological field. Int J Urol. 2007; 14:890–909.18. Chenoweth CE, Gould CV, Saint S. Diagnosis, management, and prevention of catheter-associated urinary tract infections. Infect Dis Clin North Am. 2014; 28:105–119.19. George AK, Srinivasan AK, Cho J, Sadek MA, Kavoussi LR. Surgical site infection rates following laparoscopic urological procedures. J Urol. 2011; 185:1289–1293.20. Tanner J, Dumville JC, Norman G, Fortnam M. Surgical hand antisepsis to reduce surgical site infection. Cochrane Database Syst Rev. 2016; (1):CD004288.21. Kyoda Y, Takahashi S, Takeyama K, Masumori N, Tsukamoto T. Decrease in incidence of surgical site infections in contemporary series of patients with radical cystectomy. J Infect Chemother. 2010; 16:118–122.22. Tan HJ, Wolf JS Jr, Ye Z, Wei JT, Miller DC. Complications and failure to rescue after laparoscopic versus open radical nephrectomy. J Urol. 2011; 186:1254–1260.23. Tandogdu Z, Cek M, Wagenlehner F, Naber K, Tenke P, van Ostrum E, et al. Resistance patterns of nosocomial urinary tract infections in urology departments: 8-year results of the global prevalence of infections in urology study. World J Urol. 2014; 32:791–801.24. Rosselló-Urgell J, Vaqué-Rafart J, Hermosilla-Pérez E, Allepuz-Palau A. EPINE Working Group. An approach to the study of potentially preventable nosocomial infections. Infect Control Hosp Epidemiol. 2004; 25:41–46.25. Chang R, Greene MT, Chenoweth CE, Kuhn L, Shuman E, Rogers MA, et al. Epidemiology of hospital-acquired urinary tract-related bloodstream infection at a university hospital. Infect Control Hosp Epidemiol. 2011; 32:1127–1129.26. Poulsen LL, Bisgaard M, Son NT, Trung NV, An HM, Dalsgaard A. Enterococcus and Streptococcus spp. associated with chronic and self-medicated urinary tract infections in Vietnam. BMC Infect Dis. 2012; 12:320.27. Das RN, Chandrashekhar TS, Joshi HS, Gurung M, Shrestha N, Shivananda PG. Frequency and susceptibility profile of pathogens causing urinary tract infections at a tertiary care hospital in western Nepal. Singapore Med J. 2006; 47:281–285.28. Howard AJ, Magee JT, Fitzgerald KA, Dunstan FD. Welsh Antibiotic Study Group. Factors associated with antibiotic resistance in coliform organisms from community urinary tract infection in Wales. J Antimicrob Chemother. 2001; 47:305–313.29. Briongos-Figuero LS, Gómez-Traveso T, Bachiller-Luque P, Domínguez-Gil González M, Gómez-Nieto A, Palacios-Martín T, et al. Epidemiology, risk factors and comorbidity for urinary tract infections caused by extended-spectrum beta-lactamase (ESBL)-producing enterobacteria. Int J Clin Pract. 2012; 66:891–896.30. Medina-Polo J, Arrébola-Pajares A, Pérez-Cadavid S, Benítez-Sala R, Sopeña-Sutil R, Lara-Isla A, et al. Extended-spectrum beta-lactamase-producing bacteria in a Urology ward: epidemiology, risk factors and antimicrobial susceptibility patterns. Urol Int. 2015; 95:288–292.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Infection Control for Healthcare-Associated Infections in Pediatric Patients
- Healthcare-Associated Urinary Tract Infection: Multi Drug Resistance and Risk Factors
- A Prospective Cohort Study on Predictive Risk Factors Causing Metabolic Syndrome within the First Two Years
- The clinical and microbial characteristics of healthcare-associated pneumonia
- Analysis of Characteristics and Prognosis of Healthcare-Associated Secondary Bloodstream Infection