Blood Res.
2016 Dec;51(4):225-232. 10.5045/br.2016.51.4.225.
Mesenchymal stromal cells in myeloid malignancies
- Affiliations
-
- 1Department of Hematology, Oncology and Clinical Immunology, University of Duesseldorf, Medical Faculty, Düesseldorf, Germany. thomas.schroeder@med.uni-duesseldorf.de
- KMID: 2364312
- DOI: http://doi.org/10.5045/br.2016.51.4.225
Abstract
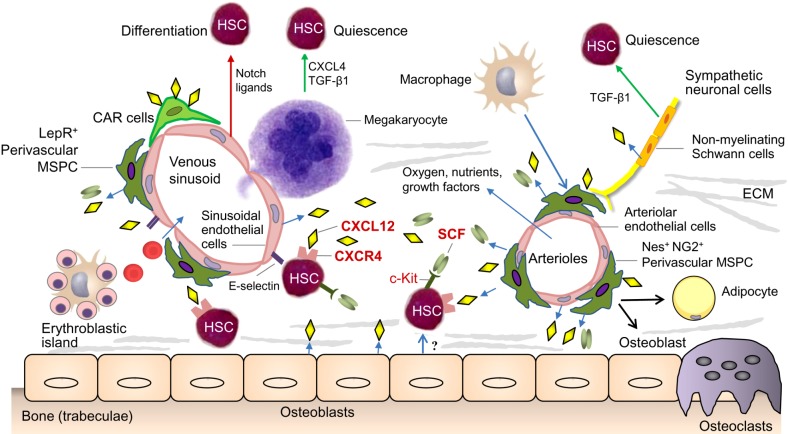
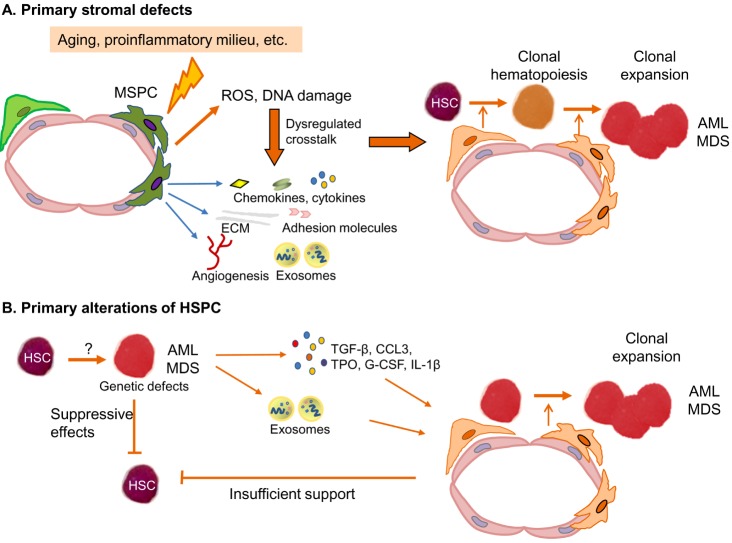
- Myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) are clonal myeloid disorders characterized by hematopoietic insufficiency. As MDS and AML are considered to originate from genetic and molecular defects of hematopoietic stem and progenitor cells (HSPC), the main focus of research in this field has focused on the characterization of these cells. Recently, the contribution of BM microenvironment to the pathogenesis of myeloid malignancies, in particular MDS and AML has gained more interest. This is based on a better understanding of its physiological role in the regulation of hematopoiesis. Additionally, it was demonstrated as a "˜proof of principle' that genetic disruption of cells of the mesenchymal or osteoblastic lineage can induce MDS, MPS or AML in mice. In this review, we summarize the current knowledge about the contribution of the BM microenvironment, in particular mesenchymal stromal cells (MSC) to the pathogenesis of AML and MDS. Furthermore, potential models integrating the BM microenvironment into the pathophysiology of these myeloid disorders are discussed. Finally, strategies to therapeutically exploit this knowledge and to interfere with the crosstalk between clonal hematopoietic cells and altered stem cell niches are introduced.
Keyword
MeSH Terms
Figure
Reference
-
1. Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013; 31:285–316. PMID: 23298209.
Article2. Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010; 466:829–834. PMID: 20703299.
Article3. Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978; 4:7–25. PMID: 747780.4. Boulais PE, Frenette PS. Making sense of hematopoietic stem cell niches. Blood. 2015; 125:2621–2629. PMID: 25762174.
Article5. Krause DS, Scadden DT. A hostel for the hostile: the bone marrow niche in hematologic neoplasms. Haematologica. 2015; 100:1376–1387. PMID: 26521296.
Article6. Schepers K, Campbell TB, Passegué E. Normal and leukemic stem cell niches: insights and therapeutic opportunities. Cell Stem Cell. 2015; 16:254–267. PMID: 25748932.
Article7. Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968; 6:230–247. PMID: 5654088.8. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8:315–317. PMID: 16923606.
Article9. Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015; 373:1136–1152. PMID: 26376137.
Article10. Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015; 126:9–16. PMID: 25931582.
Article11. Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013; 368:2059–2074. PMID: 23634996.12. Haferlach T, Nagata Y, Grossmann V, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia. 2014; 28:241–247. PMID: 24220272.
Article13. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016; 374:2209–2221. PMID: 27276561.
Article14. Eriksson A, Lennartsson A, Lehmann S. Epigenetic aberrations in acute myeloid leukemia: Early key events during leukemogenesis. Exp Hematol. 2015; 43:609–624. PMID: 26118500.
Article15. Solary E, Bernard OA, Tefferi A, Fuks F, Vainchenker W. The Ten-Eleven Translocation-2 (TET2) gene in hematopoiesis and hematopoietic diseases. Leukemia. 2014; 28:485–496. PMID: 24220273.
Article16. Kim YW, Koo BK, Jeong HW, et al. Defective Notch activation in microenvironment leads to myeloproliferative disease. Blood. 2008; 112:4628–4638. PMID: 18818392.
Article17. Rupec RA, Jundt F, Rebholz B, et al. Stroma-mediated dysregulation of myelopoiesis in mice lacking I kappa B alpha. Immunity. 2005; 22:479–491. PMID: 15845452.18. Walkley CR, Olsen GH, Dworkin S, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007; 129:1097–1110. PMID: 17574023.19. Walkley CR, Shea JM, Sims NA, Purton LE, Orkin SH. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007; 129:1081–1095. PMID: 17574022.20. Raaijmakers MH, Mukherjee S, Guo S, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010; 464:852–857. PMID: 20305640.
Article21. Geyh S, Oz S, Cadeddu RP, et al. Insufficient stromal support in MDS results from molecular and functional deficits of mesenchymal stromal cells. Leukemia. 2013; 27:1841–1851. PMID: 23797473.
Article22. Santamaría C, Muntión S, Rosón B, et al. Impaired expression of DICER, DROSHA, SBDS and some microRNAs in mesenchymal stromal cells from myelodysplastic syndrome patients. Haematologica. 2012; 97:1218–1224. PMID: 22371183.
Article23. Zhao Y, Wu D, Fei C, et al. Down-regulation of Dicer1 promotes cellular senescence and decreases the differentiation and stem cell-supporting capacities of mesenchymal stromal cells in patients with myelodysplastic syndrome. Haematologica. 2015; 100:194–204. PMID: 25361944.
Article24. Zambetti NA, Ping Z, Chen S, et al. Mesenchymal inflammation drives genotoxic stress in hematopoietic stem cells and predicts disease evolution in human pre-leukemia. Cell Stem Cell. 2016; 19:613–627. PMID: 27666011.
Article25. Basiorka AA, McGraw KL, Eksioglu EA, et al. The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood. 2016; [Epub ahead of print].
Article26. Chen X, Eksioglu EA, Zhou J, et al. Induction of myelodysplasia by myeloid-derived suppressor cells. J Clin Invest. 2013; 123:4595–4611. PMID: 24216507.
Article27. Schneider RK, Schenone M, Ferreira MV, et al. Rps14 haploinsufficiency causes a block in erythroid differentiation mediated by S100A8 and S100A9. Nat Med. 2016; 22:288–297. PMID: 26878232.
Article28. Kode A, Manavalan JS, Mosialou I, et al. Leukaemogenesis induced by an activating β-catenin mutation in osteoblasts. Nature. 2014; 506:240–244. PMID: 24429522.
Article29. Geyh S, Rodríguez-Paredes M, Jäger P, et al. Functional inhibition of mesenchymal stromal cells in acute myeloid leukemia. Leukemia. 2016; 30:683–691. PMID: 26601782.
Article30. Frisch BJ, Ashton JM, Xing L, Becker MW, Jordan CT, Calvi LM. Functional inhibition of osteoblastic cells in an in vivo mouse model of myeloid leukemia. Blood. 2012; 119:540–550. PMID: 21957195.
Article31. Schepers K, Pietras EM, Reynaud D, et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell. 2013; 13:285–299. PMID: 23850243.
Article32. Zhang B, Ho YW, Huang Q, et al. Altered microenvironmental regulation of leukemic and normal stem cells in chronic myelogenous leukemia. Cancer Cell. 2012; 21:577–592. PMID: 22516264.
Article33. Arranz L, Sánchez-Aguilera A, Martín-Pérez D, et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014; 512:78–81. PMID: 25043017.
Article34. Hanoun M, Zhang D, Mizoguchi T, et al. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell. 2014; 15:365–375. PMID: 25017722.
Article35. Blau O, Baldus CD, Hofmann WK, et al. Mesenchymal stromal cells of myelodysplastic syndrome and acute myeloid leukemia patients have distinct genetic abnormalities compared with leukemic blasts. Blood. 2011; 118:5583–5592. PMID: 21948175.
Article36. Aanei CM, Eloae FZ, Flandrin-Gresta P, et al. Focal adhesion protein abnormalities in myelodysplastic mesenchymal stromal cells. Exp Cell Res. 2011; 317:2616–2629. PMID: 21871449.
Article37. Aanei CM, Flandrin P, Eloae FZ, et al. Intrinsic growth deficiencies of mesenchymal stromal cells in myelodysplastic syndromes. Stem Cells Dev. 2012; 21:1604–1615. PMID: 21933023.
Article38. Alvi S, Shaher A, Shetty V, et al. Successful establishment of long-term bone marrow cultures in 103 patients with myelodysplastic syndromes. Leuk Res. 2001; 25:941–954. PMID: 11597729.
Article39. Deeg HJ, Beckham C, Loken MR, et al. Negative regulators of hemopoiesis and stroma function in patients with myelodysplastic syndrome. Leuk Lymphoma. 2000; 37:405–414. PMID: 10752992.
Article40. Flores-Figueroa E, Arana-Trejo RM, Gutiérrez-Espíndola G, Pérez-Cabrera A, Mayani H. Mesenchymal stem cells in myelodysplastic syndromes: phenotypic and cytogenetic characterization. Leuk Res. 2005; 29:215–224. PMID: 15607371.
Article41. Flores-Figueroa E, Montesinos JJ, Flores-Guzmán P, et al. Functional analysis of myelodysplastic syndromes-derived mesenchymal stem cells. Leuk Res. 2008; 32:1407–1416. PMID: 18405968.
Article42. Klaus M, Stavroulaki E, Kastrinaki MC, et al. Reserves, functional, immunoregulatory, and cytogenetic properties of bone marrow mesenchymal stem cells in patients with myelodysplastic syndromes. Stem Cells Dev. 2010; 19:1043–1054. PMID: 19788374.
Article43. Lopez-Villar O, Garcia JL, Sanchez-Guijo FM, et al. Both expanded and uncultured mesenchymal stem cells from MDS patients are genomically abnormal, showing a specific genetic profile for the 5q-syndrome. Leukemia. 2009; 23:664–672. PMID: 19151777.44. Raaijmakers MH. Myelodysplastic syndromes: revisiting the role of the bone marrow microenvironment in disease pathogenesis. Int J Hematol. 2012; 95:17–25. PMID: 22218882.
Article45. Soenen-Cornu V, Tourino C, Bonnet ML, et al. Mesenchymal cells generated from patients with myelodysplastic syndromes are devoid of chromosomal clonal markers and support short- and long-term hematopoiesis in vitro. Oncogene. 2005; 24:2441–2448. PMID: 15735749.
Article46. Tauro S, Hepburn MD, Peddie CM, Bowen DT, Pippard MJ. Functional disturbance of marrow stromal microenvironment in the myelodysplastic syndromes. Leukemia. 2002; 16:785–790. PMID: 11986938.
Article47. Tennant GB, Walsh V, Truran LN, Edwards P, Mills KI, Burnett AK. Abnormalities of adherent layers grown from bone marrow of patients with myelodysplasia. Br J Haematol. 2000; 111:853–862. PMID: 11122147.
Article48. Varga G, Kiss J, Várkonyi J, et al. Inappropriate Notch activity and limited mesenchymal stem cell plasticity in the bone marrow of patients with myelodysplastic syndromes. Pathol Oncol Res. 2007; 13:311–319. PMID: 18158566.
Article49. Zhao Z, Wang Z, Li Q, Li W, You Y, Zou P. The different immunoregulatory functions of mesenchymal stem cells in patients with low-risk or high-risk myelodysplastic syndromes. PLoS One. 2012; 7:e45675. PMID: 23029178.
Article50. Chandran P, Le Y, Li Y, et al. Mesenchymal stromal cells from patients with acute myeloid leukemia have altered capacity to expand differentiated hematopoietic progenitors. Leuk Res. 2015; 39:486–493. PMID: 25703353.
Article51. Chen Q, Yuan Y, Chen T. Morphology, differentiation and adhesion molecule expression changes of bone marrow mesenchymal stem cells from acute myeloid leukemia patients. Mol Med Rep. 2014; 9:293–298. PMID: 24220608.
Article52. Kim JA, Shim JS, Lee GY, et al. Microenvironmental remodeling as a parameter and prognostic factor of heterogeneous leukemogenesis in acute myelogenous leukemia. Cancer Res. 2015; 75:2222–2231. PMID: 25791383.
Article53. Zhao ZG, Liang Y, Li K, et al. Phenotypic and functional comparison of mesenchymal stem cells derived from the bone marrow of normal adults and patients with hematologic malignant diseases. Stem Cells Dev. 2007; 16:637–648. PMID: 17784837.
Article54. Ferrer RA, Wobus M, List C, et al. Mesenchymal stromal cells from patients with myelodyplastic syndrome display distinct functional alterations that are modulated by lenalidomide. Haematologica. 2013; 98:1677–1685. PMID: 23716561.
Article55. Medyouf H, Mossner M, Jann JC, et al. Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell Stem Cell. 2014; 14:824–837. PMID: 24704494.
Article56. Hertenstein B, Hambach L, Bacigalupo A, et al. Development of leukemia in donor cells after allogeneic stem cell transplantation--a survey of the European Group for Blood and Marrow Transplantation (EBMT). Haematologica. 2005; 90:969–975. PMID: 15996934.57. Churpek JE, Godley LA. How I diagnose and manage individuals at risk for inherited myeloid malignancies. Blood. 2016; [Epub ahead of print].58. Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006; 12:1167–1174. PMID: 16998484.
Article59. Krause DS, Lazarides K, von Andrian UH, Van Etten RA. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med. 2006; 12:1175–1180. PMID: 16998483.
Article60. Matsunaga T, Takemoto N, Sato T, et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med. 2003; 9:1158–1165. PMID: 12897778.
Article61. Winkler IG, Barbier V, Nowlan B, et al. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med. 2012; 18:1651–1657. PMID: 23086476.
Article62. Zeng Z, Shi YX, Samudio IJ, et al. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009; 113:6215–6224. PMID: 18955566.
Article63. Nervi B, Ramirez P, Rettig MP, et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009; 113:6206–6214. PMID: 19050309.
Article64. Uy GL, Rettig MP, Motabi IH, et al. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood. 2012; 119:3917–3924. PMID: 22308295.
Article65. Wobus M, Benath G, Ferrer RA, et al. Impact of lenalidomide on the functional properties of human mesenchymal stromal cells. Exp Hematol. 2012; 40:867–876. PMID: 22705469.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mesenchymal stem cells in acute myeloid leukemia: a focus on mechanisms involved and therapeutic concepts
- Clinical Use of Mesenchymal Stem Cells in Bone Regeneration
- mesenchymal stem cells and osteogenesis
- Reduced Osteogenic Differentiation Potential In Vivo in Acute Myeloid Leukaemia Patients Correlates with Decreased BMP4 Expression in Mesenchymal Stromal Cells
- Study on the simplifying antibody cocktail technique for isolation of human mesenchymal stromal cells (hMSCs)