J Korean Med Sci.
2017 Feb;32(2):186-194. 10.3346/jkms.2017.32.2.186.
Enhancement of Gastric Ulcer Healing and Angiogenesis by Hepatocyte Growth Factor Gene Mediated by Attenuated Salmonella in Rats
- Affiliations
-
- 1Department of Clinical Laboratory Medicine, Lanzhou General Hospital of Lanzhou Military Region, People's Liberation Army, Key Laboratory of Stem Cell and Gene Drug in Gansu Province, Lanzhou, China. haxiaoqin2013@163.com
- KMID: 2364161
- DOI: http://doi.org/10.3346/jkms.2017.32.2.186
Abstract
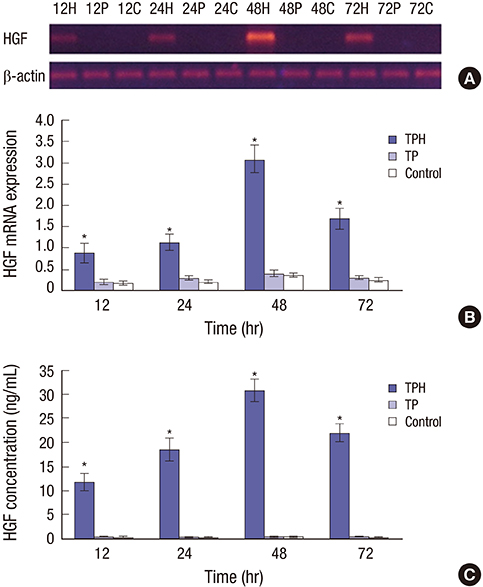
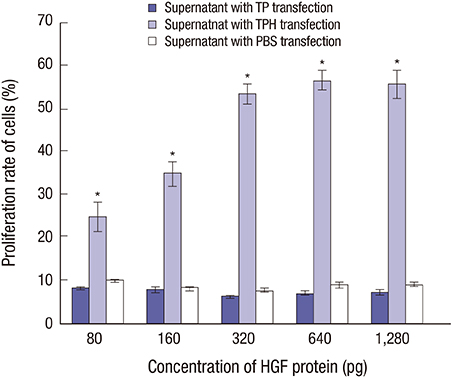
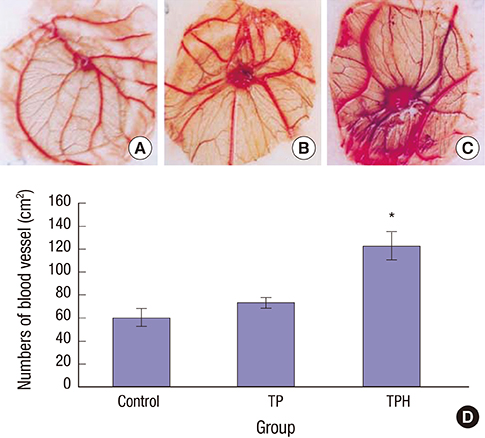
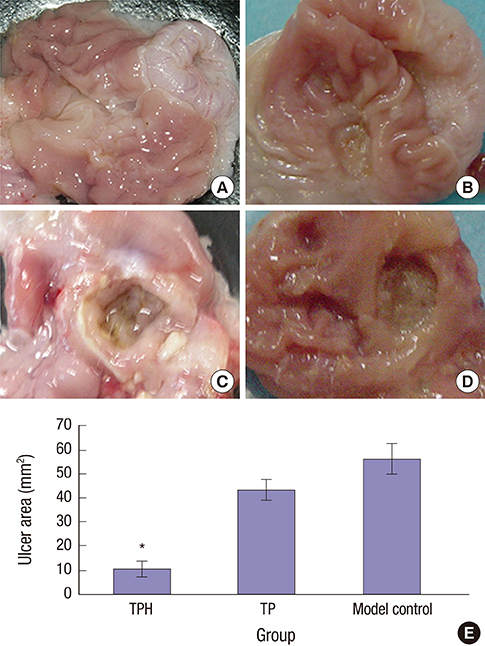
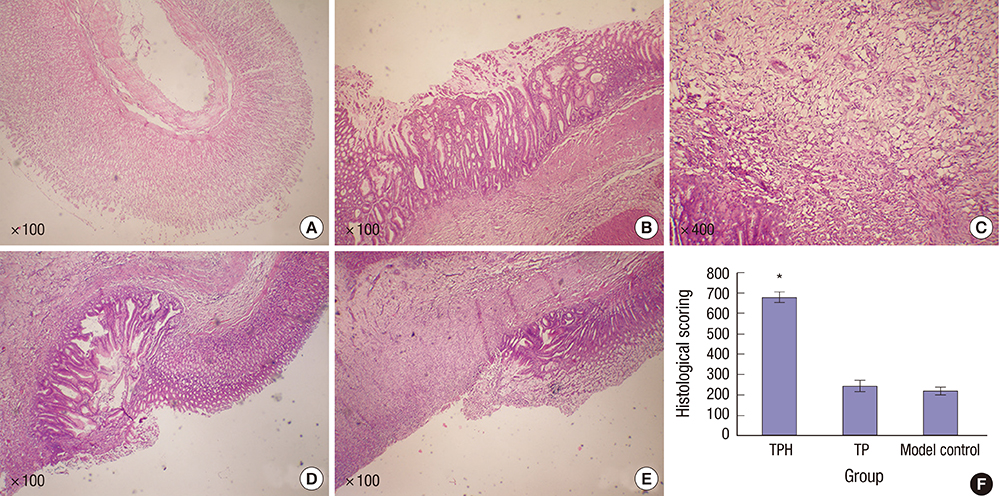
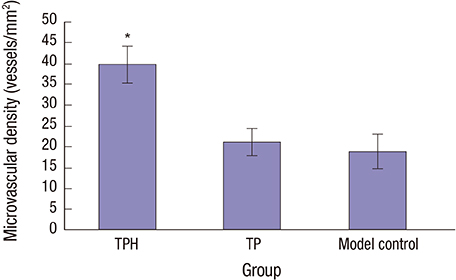
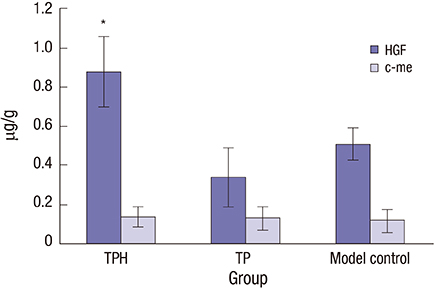
- The present study developed an oral hepatocyte growth factor (HGF) gene therapy strategy for gastric ulcers treatment. An attenuated Salmonella typhimurium that stably expressed high HGF (named as TPH) was constructed, and the antiulcerogenic effect of TPH was evaluated in a rat model of gastric ulcers that created by acetic acid subserosal injection. From day 5 after injection, TPH (1 × 10â¹ cfu), vehicle (TP, 1 × 10â¹ cfu), or sodium bicarbonate (model control) was administered orally every alternate day for three times. Then ulcer size was measured at day 21 after ulcer induction. The ulcer area in TPH-treated group was 10.56 ± 3.30 mm², which was smaller when compared with those in the TP-treated and model control groups (43.47 ± 4.18 and 56.25 ± 6.38 mm², respectively). A higher level of reepithelialization was found in TPH-treated group and the crawling length of gastric epithelial cells was significantly longer than in the other two groups (P < 0.05). The microvessel density in the ulcer granulation tissues of the TPH-treated rats was 39.9 vessels/mm², which was greater than in the TP-treated and model control rats, with a significant statistical difference. These results suggest that TPH treatment significantly accelerates the healing of gastric ulcers via stimulating proliferation of gastric epithelial cells and enhancing angiogenesis on gastric ulcer site.
MeSH Terms
Figure
Reference
-
1. Tanaka T, Arai M, Minemura S, Oyamada A, Saito K, Jiang X, Tsuboi M, Sazuka S, Maruoka D, Matsumura T, et al. Expression level of sonic hedgehog correlated with the speed of gastric mucosa regeneration in artificial gastric ulcers. J Gastroenterol Hepatol. 2014; 29:736–741.2. Kangwan N, Park JM, Kim EH, Hahm KB. Quality of healing of gastric ulcers: natural products beyond acid suppression. World J Gastrointest Pathophysiol. 2014; 5:40–47.3. Harada S, Takeuchi T, Edogawa S, Ota K, Kojima Y, Higuchi K. The availability of prostaglandin derivatives in a treatment and prevention for gastrointestinal mucosal injury. Nihon Rinsho. 2015; 73:1153–1158.4. Sugiyama T. Mucosal protective drugs. Nihon Rinsho. 2015; 73:1147–1152.5. Samsonov AA, Grechushnikov VB, Andreev DN, Iurenev GL, Korovina TI, Lezhneva IA, Maev IV. Pharmacoeconomic evaluation of treatment in patients with Helicobacter pylori-associated diseases. Ter Arkh. 2014; 86:56–61.6. Szabo S, Vincze A. Growth factors in ulcer healing: lessons from recent studies. J Physiol Paris. 2000; 94:77–81.7. Takahashi M, Takada H, Takagi K, Kataoka S, Soma R, Kuwayama H. Gastric restitution is inhibited by dexamethasone, which is reversed by hepatocyte growth factor and rebamipide. Aliment Pharmacol Ther. 2003; 18:Suppl 1. 126–132.8. Sato T, Amano H, Ito Y, Eshima K, Minamino T, Ae T, Katada C, Ohno T, Hosono K, Suzuki T, et al. Vascular endothelial growth factor receptor 1 signaling facilitates gastric ulcer healing and angiogenesis through the upregulation of epidermal growth factor expression on VEGFR1+CXCR4+ cells recruited from bone marrow. J Gastroenterol. 2014; 49:455–469.9. Uematsu Y, Fujise N, Kohsaka K, Masunaga H, Higashio K. Effective administration route for the deleted form of hepatocyte growth factor to exert its pharmacological effects. J Pharm Sci. 1999; 88:131–135.10. Qiu L, Wang X, Hao H, Mu G, Dang R, Wang J, Zhang S, Du E, Yang Z. Oral administration of attenuated Salmonella typhimurium containing a DNA vaccine against rabbit haemorrhagic disease. J Virol Methods. 2013; 188:108–113.11. Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: a Laboratory Manual. New York , NY: Cold Spring Harbor Laboratory Press;1989.12. Bai LY, Liang AX, Zhang J, Yang FF, Han L, Huo LJ, Yang LG. Effects of immunization against a DNA vaccine encoding somatostatin gene (pGM-CSF/SS) by attenuated Salmonella typhimurium on growth, reproduction and lactation in female mice. Theriogenology. 2011; 75:155–163.13. Dusseau JW, Hutchins PM. Microvascular responses to chronic hypoxia by the chick chorioallantoic membrane: a morphometric analysis. Microvasc Res. 1989; 37:138–147.14. Okabe S, Amagase K. An overview of acetic acid ulcer models--the history and state of the art of peptic ulcer research. Biol Pharm Bull. 2005; 28:1321–1341.15. Kang JM, Kim N, Kim B, Kim JH, Lee BY, Park JH, Lee MK, Lee HS, Kim JS, Jung HC, et al. Enhancement of gastric ulcer healing and angiogenesis by cochinchina Momordica seed extract in rats. J Korean Med Sci. 2010; 25:875–881.16. Weidner N, Gasparini G. Determination of epidermal growth factor receptor provides additional prognostic information to measuring tumor angiogenesis in breast carcinoma patients. Breast Cancer Res Treat. 1994; 29:97–107.17. Li J, Zheng CQ, Li Y, Yang C, Lin H, Duan HG. Hepatocyte growth factor gene-modified mesenchymal stem cells augment sinonasal wound healing. Stem Cells Dev. 2015; 24:1817–1830.18. Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984; 122:1450–1459.19. Kaido T, Imamura M. Hepatocyte growth factor: clinical implications in hepatobiliary pancreatic surgery. J Hepatobiliary Pancreat Surg. 2001; 8:65–75.20. Li Z, Yin PH, Yang SS, Li QY, Chang T, Fang L, Shi LX, Fang GE. Recombinant attenuated Salmonella typhimurium carrying a plasmid co-expressing ENDO-VEGI151 and survivin siRNA inhibits the growth of breast cancer in vivo. Mol Med Rep. 2013; 7:1215–1222.21. van Golde J, Mulder T, Blanco CE. Changes in mean chorioallantoic artery blood flow and heart rate produced by hypoxia in the developing chick embryo. Pediatr Res. 1997; 42:293–298.22. Staton CA, Stribbling SM, Tazzyman S, Hughes R, Brown NJ, Lewis CE. Current methods for assaying angiogenesis in vitro and in vivo. Int J Exp Pathol. 2004; 85:233–248.23. Nowak-Sliwinska P, Ballini JP, Wagnières G, van den Bergh H. Processing of fluorescence angiograms for the quantification of vascular effects induced by anti-angiogenic agents in the CAM model. Microvasc Res. 2010; 79:21–28.24. Favia G, Mariggio MA, Maiorano F, Cassano A, Capodiferro S, Ribatti D. Accelerated wound healing of oral soft tissues and angiogenic effect induced by a pool of aminoacids combined to sodium hyaluronate (AMINOGAM). J Biol Regul Homeost Agents. 2008; 22:109–116.25. Takagi K, Okabe S, Saziki R. A new method for the production of chronic gastric ulcer in rats and the effect of several drugs on its healing. Jpn J Pharmacol. 1969; 19:418–426.26. de-Faria FM, Almeida AC, Luiz-Ferreira A, Dunder RJ, Takayama C, da Silva MS, da Silva MA, Vilegas W, Rozza AL, Pellizzon CH, et al. Mechanisms of action underlying the gastric antiulcer activity of the Rhizophora mangle L. J Ethnopharmacol. 2012; 139:234–243.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Enhancement of Gastric Ulcer Healing and Angiogenesis by Cochinchina Momordica Seed Extract in Rats
- Therapeutic Angiogenesis with Somatic Stem Cell Transplantation
- The Prognostic Value of Tumor Angiogenesis, Hepatocyte Growth Factor and c-met Expression in Renal Cell Carcinoma
- Changes of the Serum Hepatocyte Growth Factor Levels after Hepatectomy
- Expression of Epidermal Growth Factor at Ulcer Margin and Antral Mucosa in Patients with Gastric Ulcer