J Vet Sci.
2016 Mar;17(1):53-61. 10.4142/jvs.2016.17.1.53.
Agmatine protection against chlorpromazine-induced forebrain cortex injury in rats
- Affiliations
-
- 1Military Medical Center "Karaburma", 11000 Belgrade, Serbia. bracadejanovic@yahoo.com
- 2Institute for Medical Research, Military Medical Academy, 11000 Belgrade, Serbia.
- 3Institute for Biochemistry, Faculty of Medicine, University of Nis, 18000 Nis, Serbia.
- 4Institute for Biological Research "Sinisa Stankovic", University of Belgrade, 11000 Belgrade, Serbia.
- 5Institute of Meat Hygiene and Technology, 11000 Belgrade, Serbia.
- 6Department of Obstetrics, Faculty of Veterinary Medicine, 11000 Belgrade, Serbia.
- KMID: 2363335
- DOI: http://doi.org/10.4142/jvs.2016.17.1.53
Abstract
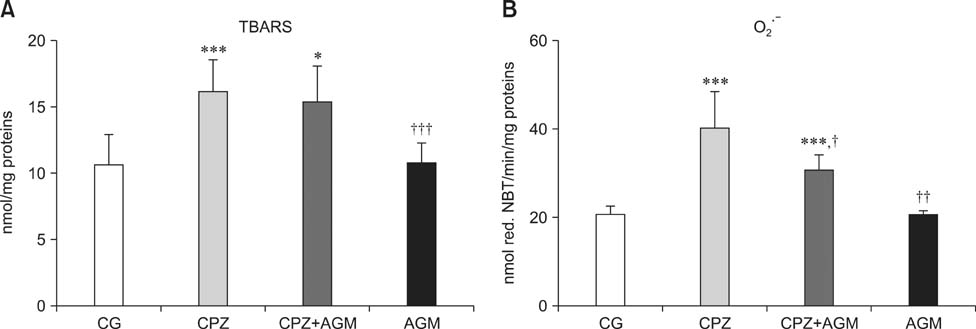
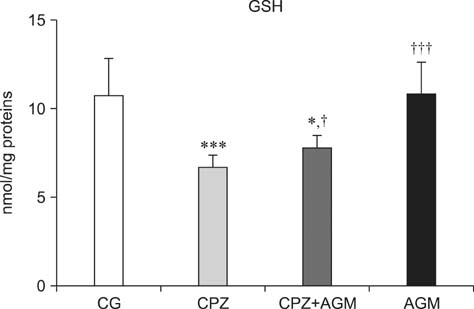
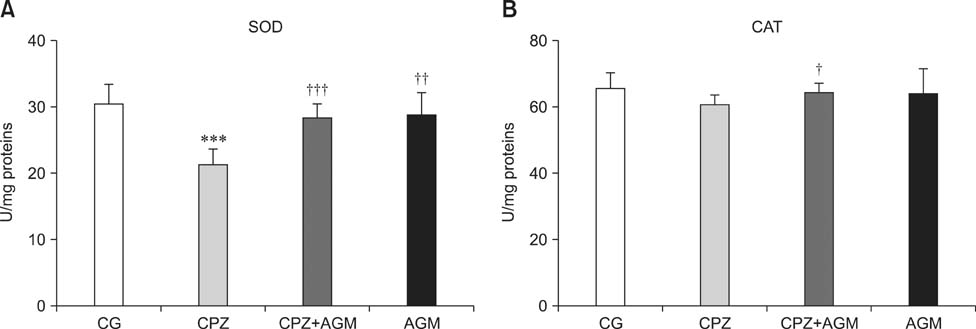
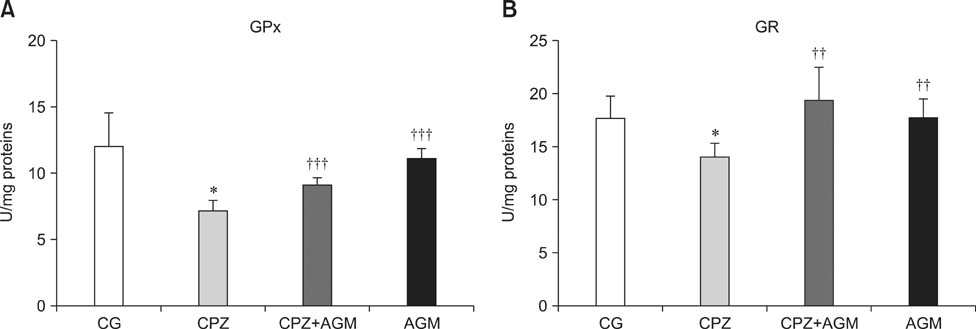
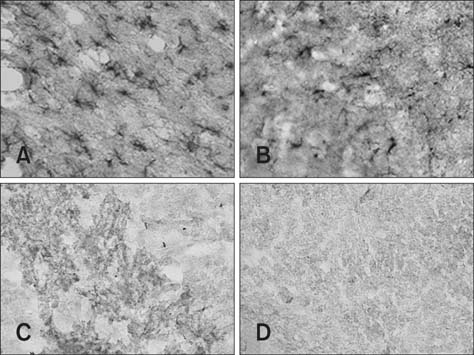
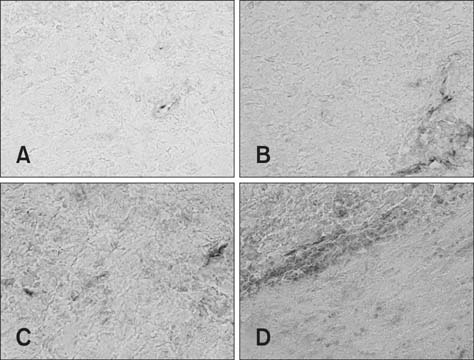
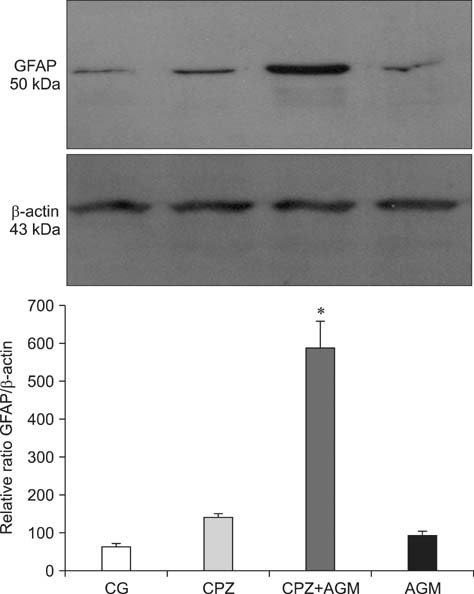
- This study was conducted to investigate whether agmatine (AGM) provides protection against oxidative stress induced by treatment with chlorpromazine (CPZ) in Wistar rats. In addition, the role of reactive oxygen species and efficiency of antioxidant protection in the brain homogenates of forebrain cortexes prepared 48 h after treatment were investigated. Chlorpromazine was applied intraperitoneally (i.p.) in single dose of 38.7 mg/kg body weight (BW) The second group was treated with both CPZ and AGM (75 mg/kg BW). The control group was treated with 0.9% saline solution in the same manner. All tested compounds were administered i.p. in a single dose. Rats were sacrificed by decapitation 48 h after treatment Treatment with AGM significantly attenuated the oxidative stress parameters and restored antioxidant capacity in the forebrain cortex. The data indicated that i.p. administered AGM exerted antioxidant action in CPZ-treated animals. Moreover, reactive astrocytes and microglia may contribute to secondary nerve-cell damage and participate in the balance of destructive vs. protective actions involved in the pathogenesis after poisoning.
MeSH Terms
Figure
Cited by 1 articles
-
Role of Agmatine on Neuroglia in Central Nervous System Injury
Sumit Barua, Jong Youl Kim, Jong Eun Lee
Brain Neurorehabil. 2019;12(1):. doi: 10.12786/bn.2019.12.e2.
Reference
-
1. Ahmed Z, Shaw G, Sharma VP, Yang C, McGowan E, Dickson DW. Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J Histochem Cytochem. 2007; 55:687–700.
Article2. Anderson ME. The DTNB-GSSG reductase recycling assay for total glutathione (GSH + 1/2 GSSG). In : Greenwald RA, editor. CRC Handbook of Methods for Oxygen Radical Research. Boca Raton: CRC Press;1985. p. 319–323.3. Atif F, Yousuf S, Agrawal SK. Restraint stress-induced oxidative damage and its amelioration with selenium. Eur J Pharmacol. 2008; 600:59–63.
Article4. Auclair C, Voisin E. Nitroblue tetrazolium reduction. In : Greenwald RA, editor. CRC Handbook of Methods for Oxygen Radical Research. Boca Raton: CRC Press;1985. p. 123–132.5. Balijepalli S, Kenchappa RS, Boyd MR, Ravindranath V. Protein thiol oxidation by haloperidol results in inhibition of mitochondrial complex I in brain regions: comparison with atypical antipsychotics. Neurochem Int. 2001; 38:425–435.
Article6. Barreto G, White RE, Ouyang Y, Xu L, Giffard RG. Astrocytes: targets for neuroprotection in stroke. Cent Nerv Syst Agents Med Chem. 2011; 11:164–173.
Article7. Carvalho M, Hawksworth G, Milhazes N, Borges F, Monks TJ, Fernandes E, Carvalho F, Bastos ML. Role of metabolites in MDMA (ecstasy)-induced nephrotoxicity: an in vitro study using rat and human renal proximal tubular cells. Arch Toxicol. 2002; 76:581–588.
Article8. Chastain EM, Duncan DS, Rodgers JM, Miller SD. The role of antigen presenting cells in multiple sclerosis. Biochim Biophys Acta. 2011; 1812:265–274.
Article9. Deneke SM. Thiol-based antioxidants. Curr Top Cell Regul. 2000; 36:151–180.
Article10. DiCiero Miranda M, de Bruin VM, Vale MR, Viana GS. Lipid peroxidation and nitrite plus nitrate levels in brain tissue from patients with Alzheimer's disease. Gerontology. 2000; 46:179–184.
Article11. Dienel GA, Hertz L. Glucose and lactate metabolism during brain activation. J Neurosci Res. 2001; 66:824–838.
Article12. Fernandez-Fernandez S, Almeida A, Bolaños JP. Antioxidant and bioenergetic coupling between neurons and astrocytes. Biochem J. 2012; 443:3–11.
Article13. Freifelder D. Zonal centrifugation. Methods Enzymol. 1973; 27:140–150.14. Furuno T, Kanno T, Arita K, Asami M, Utsumi T, Doi Y, Inoue M, Utsumi K. Roles of long chain fatty acids and carnitine in mitochondrial membrane permeability transition. Biochem Pharmacol. 2001; 62:1037–1046.
Article15. Girotti MJ, Khan N, McLellan BA. Early measurement of systemic lipid peroxidation products in the plasma of major blunt trauma patients. J Trauma. 1991; 31:32–35.
Article16. Góth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991; 196:143–151.
Article17. Gurd JW, Jones LR, Mahler HR, Moore WJ. Isolation and partial characterization of rat brain synaptic membranes. J Neurochem. 1974; 22:281–290.
Article18. Harry GJ, Schmitt TJ, Gong Z, Brown H, Zawia N, Evans HL. Lead-induced alterations of glial fibrillary acidic protein (GFAP) in the developing rat brain. Toxicol Appl Pharmacol. 1996; 139:84–93.
Article19. Heitzman RJ, Hibbitt KG, Mather I. Changes in hormone and metabolites concentrations in the blood of cows during the experimental induction of ketosis. Br Vet J. 1972; 128:347–354.
Article20. Kawai Y, Smedsrød B, Elvevold K, Wake K. Uptake of lithium carmine by sinusoidal endothelial and Kupffer cells of the rat liver: new insights into the classical vital staining and the reticulo-endothelial system. Cell Tissue Res. 1998; 292:395–410.
Article21. Khatua AK, Bhattacharyya M. NADPH-induced oxidative damage of rat liver microsomes: protective role of chlorpromazine and trifluoperazine. Pol J Pharmacol. 2001; 53:629–634.22. Levin G, Cogan U, Levy Y, Mokady S. Riboflavin deficiency and the function and fluidity of rat erythrocyte membranes. J Nutr. 1990; 120:857–861.
Article23. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951; 193:265–275.
Article24. Magliozzi R, Howell OW, Reeves C, Roncaroli F, Nicholas R, Serafini B, Aloisi F, Reynolds R. A gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol. 2010; 68:477–493.
Article25. Makarov VA, Petrukhina GN, Remov MN, Miftakhova NT, Kuz'mich MK, Popov VG. Effect of combined administration of acetylsalicylic acid and antioxidants on cellular and plasma hemostasis. Eksp Klin Farmakol. 2002; 65:32–36.26. Naidu PS, Singh A, Kulkarni SK. Carvedilol attenuates neuroleptic-induced orofacial dyskinesia: possible antioxidant mechanisms. Br J Pharmacol. 2002; 136:193–200.
Article27. Nemmiche S, Chabane-Sari D, Kadri M, Guiraud P. Cadmium-induced apoptosis in the BJAB human B cell line: involvement of PKC/ERK1/2/JNK signaling pathways in HO-1 expression. Toxicology. 2012; 300:103–111.
Article28. Nicotera P, Bellomo G, Orrenius S. The role of Ca2+ in cell killing. Chem Res Toxicol. 1990; 3:484–494.29. Ohyashiki T, Sakata N, Matsui K. Decrease of lipid fluidity of the porcine intestinal brush-border membranes by treatment with malondialdehyde. J Biochem. 1992; 111:419–423.
Article30. Parikh V, Khan MM, Mahadik SP. Differential effects of antipsychotics on expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J Psychiatr Res. 2003; 37:43–51.
Article31. Patel MX, Arista IA, Taylor M, Barnes TR. How to compare doses of different antipsychotics: a systematic review of methods. Schizophr Res. 2013; 149:141–148.
Article32. Piletz JE, Aricioglu F, Cheng JT, Fairbanks CA, Gilad VH, Haenisch B, Halaris A, Hong S, Lee JE, Li J, Liu P, Molderings GJ, Rodrigues ALS, Satriano J, Seong GJ, Wilcox G, Wu N, Gilad GM. Agmatine: clinical applications after 100 years in translation. Drug Discov Today. 2013; 18:880–893.
Article33. Reddy RD, Yao JK. Free radical pathology in schizophrenia: a review. Prostaglandins Leukot Essent Fatty Acids. 1996; 55:33–43.
Article34. Röhl C, Armbrust E, Herbst E, Jess A, Gülden M, Maser E, Rimbach G, Bösch-Saadatmandi C. Mechanisms involved in the modulation of astroglial resistance to oxidative stress induced by activated microglia: antioxidative systems, peroxide elimination, radical generation, lipid peroxidation. Neurotox Res. 2010; 17:317–331.
Article35. Rushaidhi M, Zhang H, Liu P. Effects of prolonged agmatine treatment in aged male Sprague-Dawley rats. Neuroscience. 2013; 234:116–124.
Article36. Schubert P, Ogata T, Marchini C, Ferroni S. Glia-related pathomechanisms in Alzheimer's disease: a therapeutic target? Mech Ageing Dev. 2001; 123:47–57.
Article37. Schwartz PJ, Reaume A, Scott R, Coyle JT. Effects of over- and under-expression of Cu,Zn-superoxide dismutase on the toxicity of glutamate analogs in transgenic mouse striatum. Brain Res. 1998; 789:32–39.
Article38. Sulaiman AA, Al-Shawi NN, Jwaied AH, Mahmood DM, Hussain SA. Protective effect of melatonin against chlorpromazine-induced liver disease in rats. Saudi Med J. 2006; 27:1477–1482.39. Sun M, Zigman S. An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Anal Biochem. 1978; 90:81–89.
Article40. Todorova K, Ivanov S, Genova M. Selenium and glutathion peroxidase enzyme levels in diabetic patients with early spontaneous abortions. Akush Ginekol (Sofiia). 2006; 45:3–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Quantitative Analysis of Agmatine by HPLC in Ischemic Brain
- Agmatine Attenuates Brain Edema and Apoptotic Cell Death after Traumatic Brain Injury
- The Effect of Agmatine on Expression of MMP2 and MMP9 in Cerebral Ischemia
- Effect of a Rostral Basal Forebrain Lesion on Neuropeptide Containing Neurons in the Rat Cerebral Cortex
- Regulation of endothelial nitric oxide synthase by agmatine after transient global cerebral ischemia in rat brain