J Korean Med Sci.
2015 Dec;30(12):1856-1864. 10.3346/jkms.2015.30.12.1856.
The Toxicity of Nonsteroidal Anti-inflammatory Eye Drops against Human Corneal Epithelial Cells in Vitro
- Affiliations
-
- 1Department of Ophthalmology, Pusan National University School of Medicine and Medical Research Institute, Pusan National University Hospital, Busan, Korea. jongsool@pusan.ac.kr
- 2B&G Eye Clinic, Busan, Korea.
- 3Department of Ophthalmology, Pusan National University School of Medicine and Medical Research Institute, Yangsan Pusan National University Hospital, Yangsan, Korea.
- KMID: 2359972
- DOI: http://doi.org/10.3346/jkms.2015.30.12.1856
Abstract
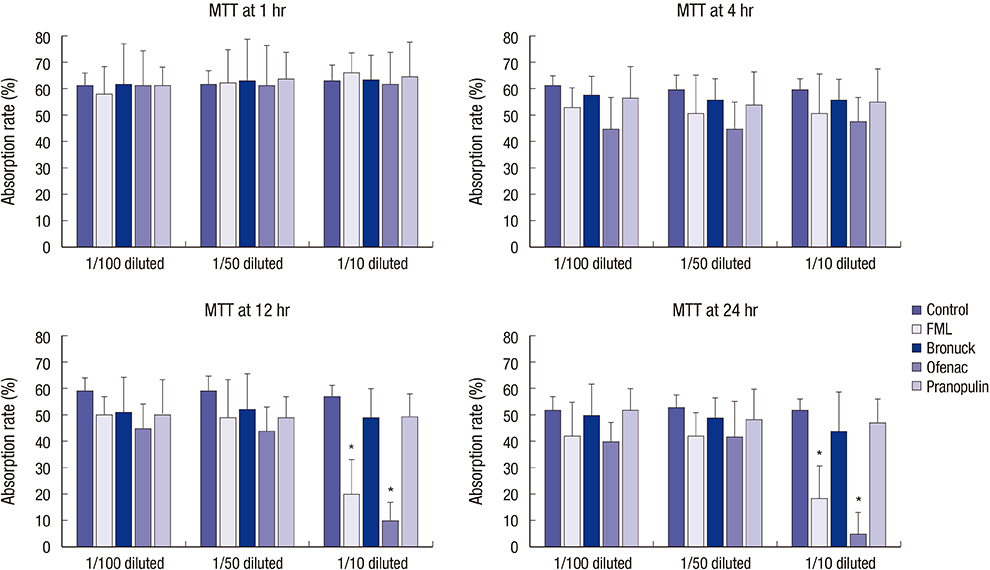
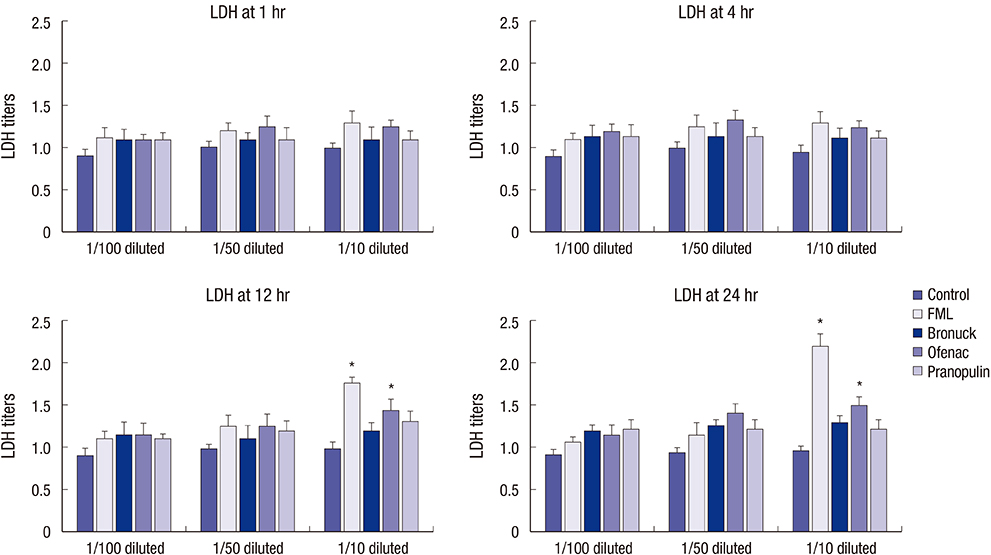
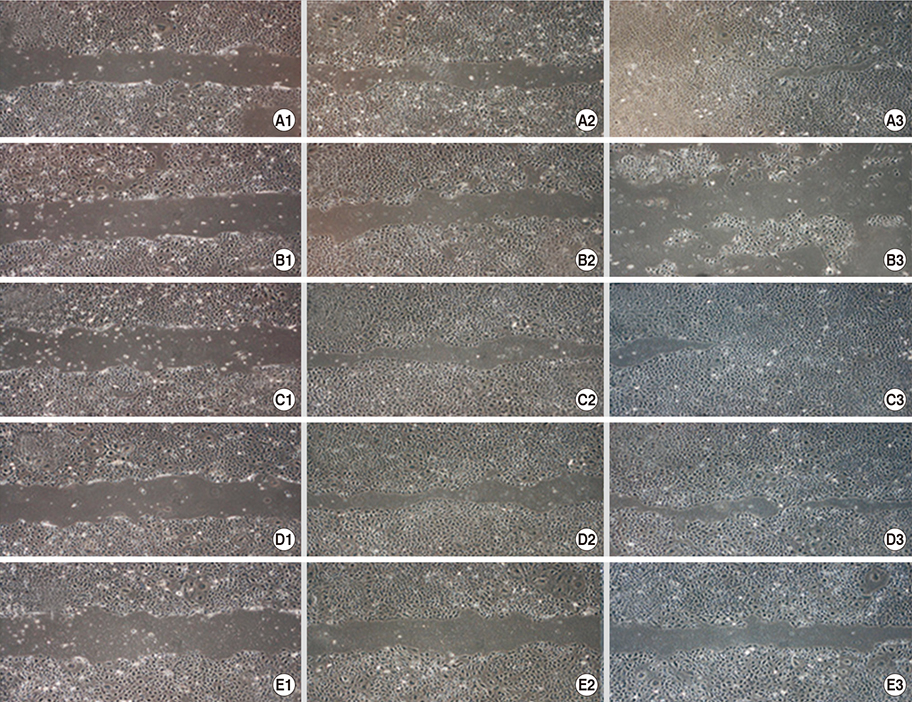
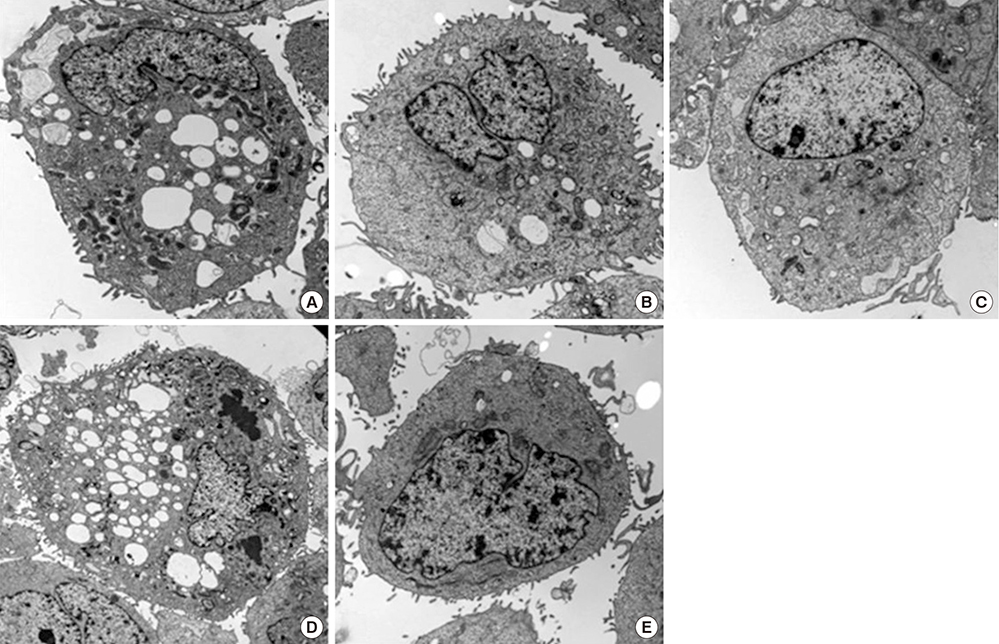
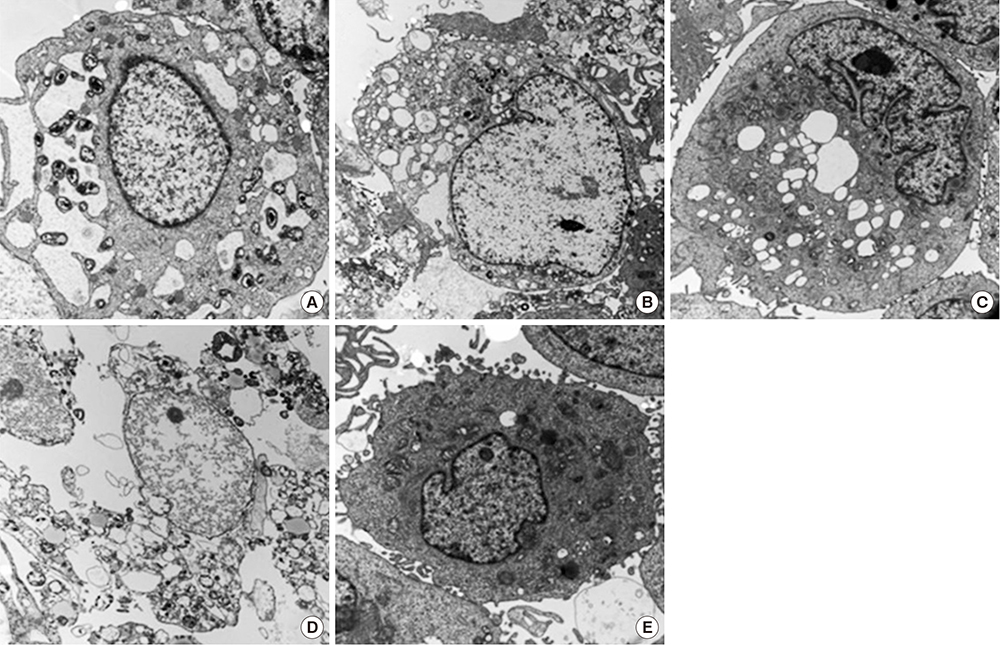
- This study investigated the toxicity of commercial non-steroid anti-inflammatory drug (NSAID) eye solutions against corneal epithelial cells in vitro. The biologic effects of 1/100-, 1/50-, and 1/10-diluted bromfenac sodium, pranoprofen, diclofenac sodium, and the fluorometholone on corneal epithelial cells were evaluated after 1-, 4-, 12-, and 24-hr of exposure compared to corneal epithelial cell treated with balanced salt solution as control. Cellular metabolic activity, cellular damage, and morphology were assessed. Corneal epithelial cell migration was quantified by the scratch-wound assay. Compared to bromfenac and pranoprofen, the cellular metabolic activity of diclofenac and fluorometholone significantly decreased after 12-hr exposure, which was maintained for 24-hr compared to control. Especially, at 1/10-diluted eye solution for 24-hr exposure, the LDH titers of fluorometholone and diclofenac sodium markedly increased more than those of bromfenac and pranoprofen. In diclofenac sodium, the Na+ concentration was lower and amount of preservatives was higher than other NSAIDs eye solutions tested. However, the K+ and Cl- concentration, pH, and osmolarity were similar for all NSAIDs eye solutions. Bromfenac and pranoprofen significantly promoted cell migration, and restored wound gap after 48-hr exposure, compared with that of diclofenac or fluorometholone. At 1/50-diluted eye solution for 48-hr exposure, the corneal epithelial cellular morphology of diclofenac and fluorometholone induced more damage than that of bromfenac or pranoprofen. Overall, the corneal epithelial cells in bromfenac and pranoprofen NSAID eye solutions are less damaged compared to those in diclofenac, included fluorometholone as steroid eye solution.
Keyword
MeSH Terms
-
Anti-Inflammatory Agents, Non-Steroidal/administration & dosage/*toxicity
Benzophenones/administration & dosage/toxicity
Benzopyrans/administration & dosage/toxicity
Bromobenzenes/administration & dosage/toxicity
Cell Movement/drug effects
Cells, Cultured
Diclofenac/administration & dosage/toxicity
Epithelial Cells/drug effects/metabolism/ultrastructure
Epithelium, Corneal/cytology/*drug effects/metabolism
Fluorometholone/administration & dosage/toxicity
Humans
L-Lactate Dehydrogenase/metabolism
Microscopy, Electron, Transmission
Ophthalmic Solutions
Propionates/administration & dosage/toxicity
Anti-Inflammatory Agents, Non-Steroidal
Benzophenones
Benzopyrans
Bromobenzenes
Diclofenac
Fluorometholone
L-Lactate Dehydrogenase
Ophthalmic Solutions
Propionates
Figure
Reference
-
1. Chan CK, Lam DS. The comparison of efficacies of topical corticosteroids and nonsteroidal anti-inflammatory drops on dry eye patients: a clinical and immunocytochemical study. Am J Ophthalmol. 2004; 137:1157–1158.2. McColgin AZ, Heier JS. Control of intraocular inflammation associated with cataract surgery. Curr Opin Ophthalmol. 2000; 11:3–6.3. McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002; 25:33–55.4. Yülek F, Ozdek S, Gürelik G, Hasanreisoğlu B. Effect of topical steroids on corneal epithelial healing after vitreoretinal surgery. Acta Ophthalmol Scand. 2006; 84:319–322.5. Waterbury L, Kunysz EA, Beuerman R. Effects of steroidal and non-steroidal anti-inflammatory agents on corneal wound healing. J Ocul Pharmacol. 1987; 3:43–54.6. Gasset AR, Lorenzetti DW, Ellison EM, Kaufman HE. Quantitative corticosteroid effect on corneal wound healing. Arch Ophthalmol. 1969; 81:589–591.7. Lee JS, Jung W, Choi YR, Kim YS. The effects of steroids and nonsteroidal antiinflammatory agents on proliferation of human ocular fibroblast. J Korean Ophthalmol Soc. 1999; 40:1496–1502.8. Kapin MA, Yanni JM, Brady MT, McDonough TJ, Flanagan JG, Rawji MH, Dahlin DC, Sanders ME, Gamache DA. Inflammation-mediated retinal edema in the rabbit is inhibited by topical nepafenac. Inflammation. 2003; 27:281–291.9. Gamache DA, Graff G, Brady MT, Spellman JM, Yanni JM. Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: I. Assessment of anti-inflammatory efficacy. Inflammation. 2000; 24:357–370.10. Assouline M, Renard G, Arne JL, David T, Lasmolles C, Malecaze F, Pouliquen YJ. A prospective randomized trial of topical soluble 0.1% indomethacin versus 0.1% diclofenac versus placebo for the control of pain following excimer laser photorefractive keratectomy. Ophthalmic Surg Lasers. 1998; 29:365–374.11. Lindstrom R. The pharmacologic and pathophysiologic rationale for using NSAIDs in ocular inflammatory disease and ocular surgery. Int Ophthalmol Clin. 2006; 46:7–11.12. Guidera AC, Luchs JI, Udell IJ. Keratitis, ulceration, and perforation associated with topical nonsteroidal anti-inflammatory drugs. Ophthalmology. 2001; 108:936–944.13. Hersh PS, Rice BA, Baer JC, Wells PA, Lynch SE, McGuigan LJ, Foster CS. Topical nonsteroidal agents and corneal wound healing. Arch Ophthalmol. 1990; 108:577–583.14. Ayaki M, Iwasawa A, Soda M, Yaguchi S, Koide R. Cytotoxicity of five fluoroquinolone and two nonsteroidal anti-inflammatory benzalkonium chloride-free ophthalmic solutions in four corneoconjunctival cell lines. Clin Ophthalmol. 2010; 4:1019–1024.15. Gipson IK, Grill SM. A technique for obtaining sheets of intact rabbit corneal epithelium. Invest Ophthalmol Vis Sci. 1982; 23:269–273.16. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.17. Wee WR, Wang XW, McDonnell PJ. Effect of artificial tears on cultured keratocytes in vitro. Cornea. 1995; 14:273–279.18. Park YS. Physiology of body fluid. In : Kang DH, editor. Physiology. 5th ed. Seoul: in-Kwang Publishing & Printing;2000. p. 585–606.19. Lee JS, Oum BS. The effects of artificial tear formulations and anti-inflammatory agents on the cultured keratocytes of rabbit. J Korean Ophthalmol Soc. 1998; 39:42–51.20. Ramselaar JA, Boot JP, van Haeringen NJ, van Best JA, Oosterhuis JA. Corneal epithelial permeability after instillation of ophthalmic solutions containing local anaesthetics and preservatives. Curr Eye Res. 1988; 7:947–950.21. Epstein SP, Ahdoot M, Marcus E, Asbell PA. Comparative toxicity of preservatives on immortalized corneal and conjunctival epithelial cells. J Ocul Pharmacol Ther. 2009; 25:113–119.22. Kusano M, Uematsu M, Kumagami T, Sasaki H, Kitaoka T. Evaluation of acute corneal barrier change induced by topically applied preservatives using corneal transepithelial electric resistance in vivo. Cornea. 2010; 29:80–85.23. Ku EC, Lee W, Kothari HV, Scholer DW. Effect of diclofenac sodium on the arachidonic acid cascade. Am J Med. 1986; 80:18–23.24. O'Brien TP, Li QJ, Sauerburger F, Reviglio VE, Rana T, Ashraf MF. The role of matrix metalloproteinases in ulcerative keratolysis associated with perioperative diclofenac use. Ophthalmology. 2001; 108:656–659.25. Hsu JK, Johnston WT, Read RW, McDonnell PJ, Pangalinan R, Rao N, Smith RE. Histopathology of corneal melting associated with diclofenac use after refractive surgery. J Cataract Refract Surg. 2003; 29:250–256.26. Ku EC, Kothari H, Lee W, Kimble EF, Liauw LH. Effects of diclofenac sodium on arachidonic acid metabolism. Agents Actions Suppl. 1985; 17:189–193.27. Uyemura SA, Santos AC, Mingatto FE, Jordani MC, Curti C. Diclofenac sodium and mefenamic acid: potent inducers of the membrane permeability transition in renal cortex mitochondria. Arch Biochem Biophys. 1997; 342:231–235.28. Qu M, Wang Y, Yang L, Zhou Q. Different cellular effects of four anti-inflammatory eye drops on human corneal epithelial cells: independent in active components. Mol Vis. 2011; 17:3147–3155.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mycobacterium abscessus Corneal Ulcer with Conjunctival Toxicity due to Topical Amikacin
- Effect of Preservative-Free Healon Eye Drop on Human Corneal Epithelial Cell in Vitro
- Effect of Preservative-free Artificial Eye Drop on Human Corneal Epithelial Cell in vitro
- The Antibacterial Sensitivity of Domestic Topical Antibiotics: in vitro test
- Long-Term Effect of Preservative-Free Sodium Hyaluronate Eye Drop on Human Corneal Epithelial Cell