J Korean Med Sci.
2015 Dec;30(12):1754-1763. 10.3346/jkms.2015.30.12.1754.
Human Urine-derived Stem Cells Seeded Surface Modified Composite Scaffold Grafts for Bladder Reconstruction in a Rat Model
- Affiliations
-
- 1Department of Urology, Kyungpook National University School of Medicine, Daegu, Korea. tgkwon@knu.ac.kr
- 2Bio-Medical Research Institute, Kyungpook National University Hospital, Daegu, Korea.
- 3Department of Neural Development and Disease, Korea Brain Research Institute, Daegu, Korea.
- 4Department of Laboratory Animal Research Support Team, Yeungnam University, Daegu, Korea.
- 5Department of Nanobiomedical Science & WCU Research Center, Dankook University, Cheonan, Korea.
- 6Department of Urology, Yeungnam University College of Medicine, Daegu, Korea.
- 7Department of Advanced Materials, Hannam University, Daejeon, Korea.
- 8Department of Emergency Medicine, Kyungpook National University School of Medicine, Daegu, Korea.
- KMID: 2359959
- DOI: http://doi.org/10.3346/jkms.2015.30.12.1754
Abstract
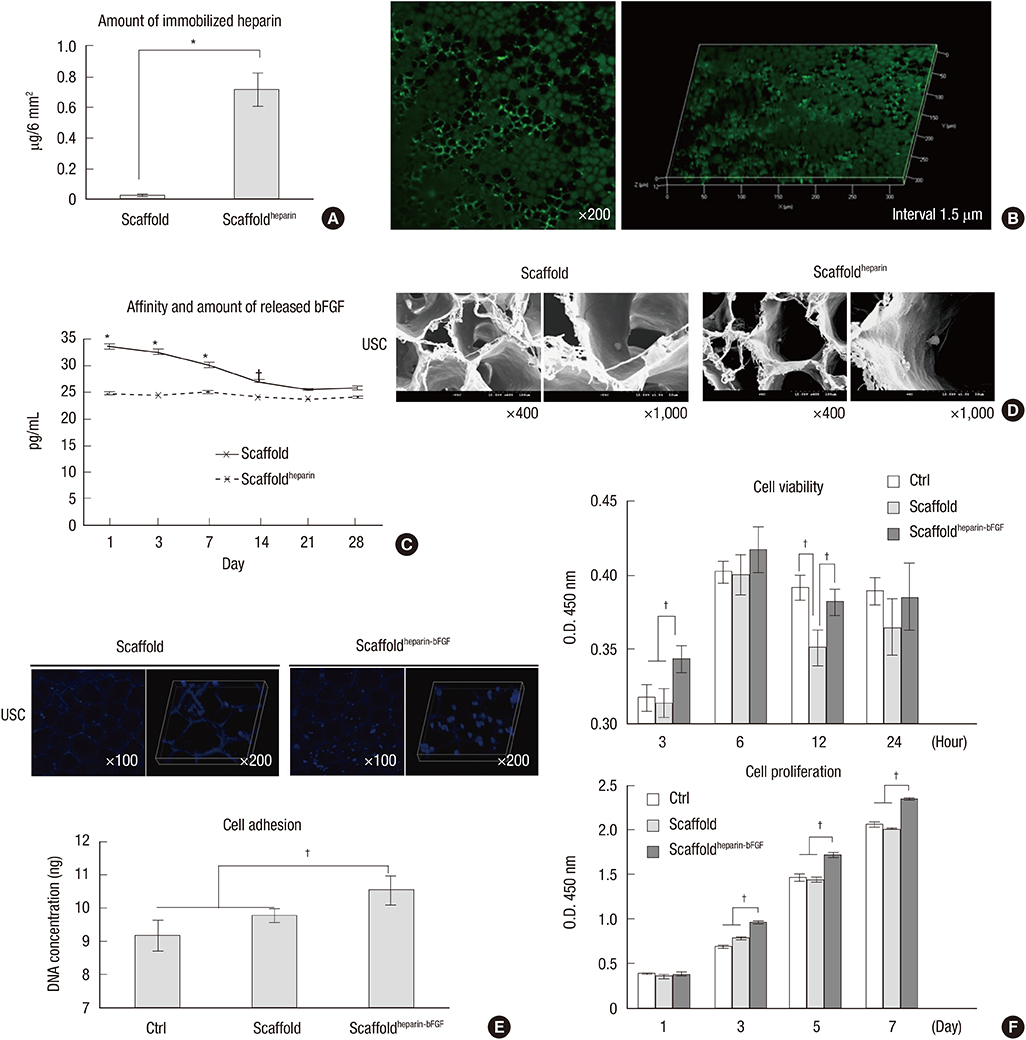
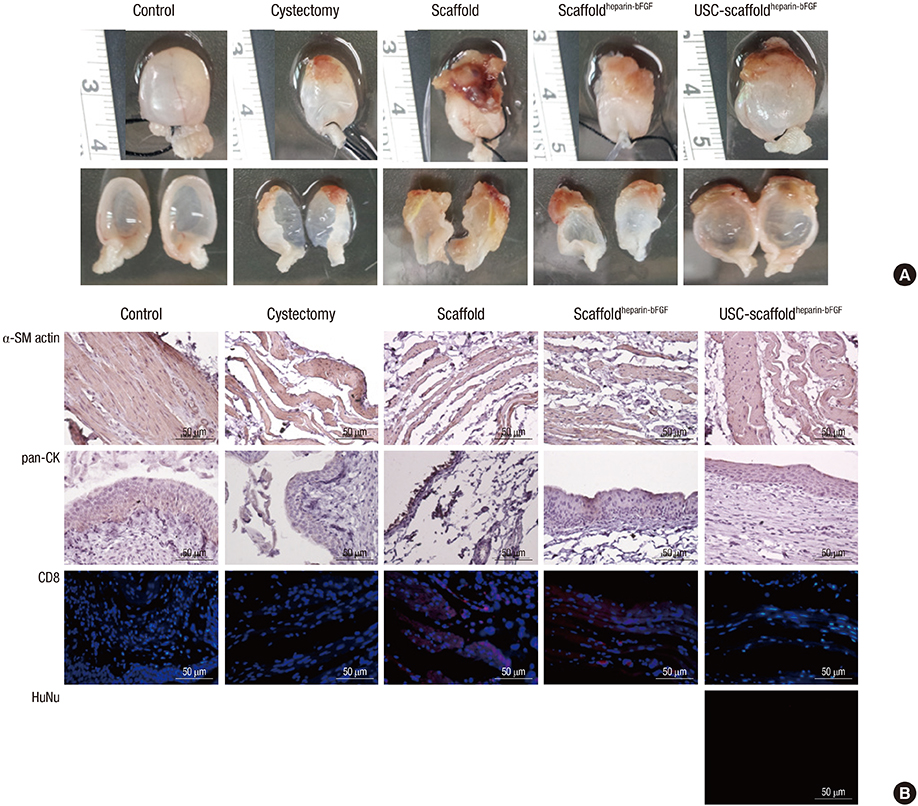
- We conducted this study to investigate the synergistic effect of human urine-derived stem cells (USCs) and surface modified composite scaffold for bladder reconstruction in a rat model. The composite scaffold (Polycaprolactone/Pluronic F127/3 wt% bladder submucosa matrix) was fabricated using an immersion precipitation method, and heparin was immobilized on the surface via covalent conjugation. Basic fibroblast growth factor (bFGF) was loaded onto the heparin-immobilized scaffold by a simple dipping method. In maximal bladder capacity and compliance analysis at 8 weeks post operation, the USCs-scaffold(heparin-bFGF) group showed significant functional improvement (2.34 ± 0.25 mL and 55.09 ± 11.81 microL/cm H2O) compared to the other groups (2.60 ± 0.23 mL and 56.14 ± 9.00 microL/cm H2O for the control group, 1.46 ± 0.18 mL and 34.27 ± 4.42 microL/cm H2O for the partial cystectomy group, 1.76 ± 0.22 mL and 35.62 ± 6.69 microL/cm H2O for the scaffold group, and 1.92 ± 0.29 mL and 40.74 ± 7.88 microL/cm H2O for the scaffold(heparin-bFGF) group, respectively). In histological and immunohistochemical analysis, the USC-scaffold(heparin-bFGF) group showed pronounced, well-differentiated, and organized smooth muscle bundle formation, a multi-layered and pan-cytokeratin-positive urothelium, and high condensation of submucosal area. The USCs seeded scaffold(heparin-bFGF) exhibits significantly increased bladder capacity, compliance, regeneration of smooth muscle tissue, multi-layered urothelium, and condensed submucosa layers at the in vivo study.
Keyword
MeSH Terms
-
Adult Stem Cells/cytology/metabolism/*transplantation
Animals
Biocompatible Materials/chemistry
Cell Differentiation
Fibroblast Growth Factor 2/administration & dosage
Heparin/administration & dosage
Humans
Materials Testing
Models, Animal
Poloxamer
Polyesters
Rats
Reconstructive Surgical Procedures
Regeneration
Tissue Engineering/*methods
Tissue Scaffolds/chemistry
Urinary Bladder/anatomy & histology/physiology/*surgery
Urine/*cytology
Biocompatible Materials
Fibroblast Growth Factor 2
Heparin
Poloxamer
Polyesters
Figure
Cited by 1 articles
-
Bladder Regeneration Using a Polycaprolactone Scaffold with a Gradient Structure and Growth Factors in a Partially Cystectomized Rat Model
Ho Yong Kim, So Young Chun, Eun Hye Lee, Bomi Kim, Yun-Sok Ha, Jae-Wook Chung, Jun Nyung Lee, Bum Soo Kim, Se Heang Oh, Tae Gyun Kwon
J Korean Med Sci. 2020;35(41):e374. doi: 10.3346/jkms.2020.35.e374.
Reference
-
1. Jednak R. The evolution of bladder augmentation: from creating a reservoir to reconstituting an organ. Front Pediatr. 2014; 2:10.2. Salem SA, Hwei NM, Bin Saim A, Ho CC, Sagap I, Singh R, Yusof MR, Md Zainuddin Z, Idrus RB. Polylactic-co-glycolic acid mesh coated with fibrin or collagen and biological adhesive substance as a prefabricated, degradable, biocompatible, and functional scaffold for regeneration of the urinary bladder wall. J Biomed Mater Res A. 2013; 101:2237–2247.3. Kim BS, Mooney DJ. Engineering smooth muscle tissue with a predefined structure. J Biomed Mater Res. 1998; 41:322–332.4. Chen W, Shi C, Yi S, Chen B, Zhang W, Fang Z, Wei Z, Jiang S, Sun X, Hou X, et al. Bladder regeneration by collagen scaffolds with collagen binding human basic fibroblast growth factor. J Urol. 2010; 183:2432–2439.5. Lanza RP, Langer RS, Vacanti JP. Principles of tissue engineering. Amsterdam: Academic Press;2013.6. Geng HQ, Tang DX, Chen F, Wu XR, Zhou X. The bladder submucosa acellular matrix as a cell deliverer in tissue engineering. World J Pediatr. 2006; 2:57–60.7. Zhang Y, Kropp BP, Lin HK, Cowan R, Cheng EY. Bladder regeneration with cell-seeded small intestinal submucosa. Tissue Eng. 2004; 10:181–187.8. Zambon JP, de Sá Barretto LS, Nakamura AN, Duailibi S, Leite K, Magalhaes RS, Orlando G, Ross CL, Peloso A, Almeida FG. Histological changes induced by Polyglycolic-Acid (PGA) scaffolds seeded with autologous adipose or muscle-derived stem cells when implanted on rabbit bladder. Organogenesis. 2014; 10:278–288.9. Gomelsky A, Dmochowski RR. Tissue Engineering for Neurogenic Bladder. In : Wein AJ, Andersson KE, Drake MJ, Dmochowski RR, editors. Bladder dysfunction in the adult : the basis for clinical management. New York, NY: Springer;2014. p. 265–276.10. Roth CC, Mondalek FG, Kibar Y, Ashley RA, Bell CH, Califano JA, Madihally SV, Frimberger D, Lin HK, Kropp BP. Bladder regeneration in a canine model using hyaluronic acid-poly(lactic-co-glycolic-acid) nanoparticle modified porcine small intestinal submucosa. BJU Int. 2011; 108:148–155.11. Yu DS, Lee CF, Chen HI, Chang SY. Bladder wall grafting in rats using salt-modified and collagen-coated polycaprolactone scaffolds: preliminary report. Int J Urol. 2007; 14:939–944.12. Jang YJ, Chun SY, Kim GN, Kim JR, Oh SH, Lee JH, Kim BS, Song PH, Yoo ES, Kwon TG. Characterization of a novel composite scaffold consisting of acellular bladder submucosa matrix, polycaprolactone and Pluronic F127 as a substance for bladder reconstruction. Acta Biomater. 2014; 10:3117–3125.13. Lee M, Wu BM, Stelzner M, Reichardt HM, Dunn JC. Intestinal smooth muscle cell maintenance by basic fibroblast growth factor. Tissue Eng Part A. 2008; 14:1395–1402.14. Yoon JJ, Chung HJ, Lee HJ, Park TG. Heparin-immobilized biodegradable scaffolds for local and sustained release of angiogenic growth factor. J Biomed Mater Res A. 2006; 79:934–942.15. Pigott JH, Ishihara A, Wellman ML, Russell DS, Bertone AL. Investigation of the immune response to autologous, allogeneic, and xenogeneic mesenchymal stem cells after intra-articular injection in horses. Vet Immunol Immunopathol. 2013; 156:99–106.16. Chun SY, Kim HT, Lee JS, Kim MJ, Kim BS, Kim BW, Kwon TG. Characterization of urine-derived cells from upper urinary tract in patients with bladder cancer. Urology. 2012; 79:1186.e1–1186.e7.17. Oh SH, Kim JR, Kwon GB, Namgung U, Song KS, Lee JH. Effect of surface pore structure of nerve guide conduit on peripheral nerve regeneration. Tissue Eng Part C Methods. 2013; 19:233–243.18. Jonnalagadda SB, Gollapalli NR. Kinetics of reduction of toluidine blue with sulfite-kinetic salt effect in elucidation of mechanism. J Chem Educ. 2000; 77:506.19. Lee AC, Yu VM, Lowe JB 3rd, Brenner MJ, Hunter DA, Mackinnon SE, Sakiyama-Elbert SE. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp Neurol. 2003; 184:295–303.20. Smith PK, Mallia AK, Hermanson GT. Colorimetric method for the assay of heparin content in immobilized heparin preparations. Anal Biochem. 1980; 109:466–473.21. Zhang Y, McNeill E, Tian H, Soker S, Andersson KE, Yoo JJ, Atala A. Urine derived cells are a potential source for urological tissue reconstruction. J Urol. 2008; 180:2226–2233.22. Jeon O, Kang SW, Lim HW, Chung JH, Kim BS. Long-term and zero-order release of basic fibroblast growth factor from heparin-conjugated poly(L-lactide-co-glycolide) nanospheres and fibrin gel. Biomaterials. 2006; 27:1598–1607.23. Atala A. Tissue engineering of human bladder. Br Med Bull. 2011; 97:81–104.24. Sasisekharan R, Ernst S, Venkataraman G. On the regulation of fibroblast growth factor activity by heparin-like glycosaminoglycans. Angiogenesis. 1997; 1:45–54.25. Wang XH, Li DP, Wang WJ, Feng QL, Cui FZ, Xu YX, Song XH. Covalent immobilization of chitosan and heparin on PLGA surface. Int J Biol Macromol. 2003; 33:95–100.26. Steffens GC, Yao C, Prével P, Markowicz M, Schenck P, Noah EM, Pallua N. Modulation of angiogenic potential of collagen matrices by covalent incorporation of heparin and loading with vascular endothelial growth factor. Tissue Eng. 2004; 10:1502–1509.27. Cai S, Liu Y, Shu XZ, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005; 26:6054–6067.28. Liu LS, Ng CK, Thompson AY, Poser JW, Spiro RC. Hyaluronate-heparin conjugate gels for the delivery of basic fibroblast growth factor (FGF-2). J Biomed Mater Res. 2002; 62:128–135.29. Kanematsu A, Yamamoto S, Noguchi T, Ozeki M, Tabata Y, Ogawa O. Bladder regeneration by bladder acellular matrix combined with sustained release of exogenous growth factor. J Urol. 2003; 170:1633–1638.30. Lech M, Gröbmayr R, Weidenbusch M, Anders HJ. Tissues use resident dendritic cells and macrophages to maintain homeostasis and to regain homeostasis upon tissue injury: the immunoregulatory role of changing tissue environments. Mediators Inflamm. 2012; 2012:951390.31. Kim BS, Chun SY, Lee JK, Lim HJ, Bae JS, Chung HY, Atala A, Soker S, Yoo JJ, Kwon TG. Human amniotic fluid stem cell injection therapy for urethral sphincter regeneration in an animal model. BMC Med. 2012; 10:94.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of serum-derived albumin scaffold and canine adipose tissue-derived mesenchymal stem cells on osteogenesis in canine segmental bone defect model
- Augmentation Cystoplasty using Hydroxapatite/chitosan Composite Sheet Seeded with Autologous Muscle-derived Stem Cells
- Stem Cells Seeded on Multilayered Scaffolds Implanted into an Injured Bladder Rat Model Improves Bladder Function
- Incorporation of Smooth Muscle Cells Derived from HumanAdipose Stem Cells on Poly(Lactic-co-Glycolic Acid) Scaffoldfor the Reconstruction of Subtotally Resected Urinary Bladderin Athymic Rats
- Osteogenic Differentiation of Human Adipose-derived Stem Cells within PLGA(Poly(D,L-lactic-co-glycolic acid)) Scaffold in the Nude Mouse