Cancer Res Treat.
2016 Oct;48(4):1363-1372. 10.4143/crt.2015.456.
Locoregional Recurrence by Tumor Biology in Breast Cancer Patients after Preoperative Chemotherapy and Breast Conservation Treatment
- Affiliations
-
- 1Center for Breast Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Korea. radiat@snu.ac.kr
- 2Department of Radiation Oncology, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 3Department of Radiation Oncology, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Radiation Oncology, Soonchunhyang University Hospital, Soonchunhyang University College of Medicine, Seoul, Korea.
- KMID: 2356238
- DOI: http://doi.org/10.4143/crt.2015.456
Abstract
- PURPOSE
The purpose of this study is to determine whether breast cancer subtype can affect locoregional recurrence (LRR) and ipsilateral breast tumor recurrence (IBTR) after neoadjuvant chemotherapy (NAC) and breast-conserving therapy (BCT).
MATERIALS AND METHODS
We evaluated 335 consecutive patients with clinical stage II-III breast cancer who received NAC plus BCT from 2002 to 2009. Patients were classified according to six molecular subtypes: luminal A (hormone receptor [HR]+/HER2-/Ki-67 < 15%, n=113), luminal B1 (HR+/HER2-/Ki-67 ≥ 15%, n=33), luminal B2 (HR+/HER2+, n=83), HER2 with trastuzumab (HER2[T+]) (HR-/HER2+/use of trastuzumab, n=14), HER2 without trastuzumab (HER2[T-]) (HR-/HER2+, n=31), and triple negative (TN) (HR-/HER2-, n=61).
RESULTS
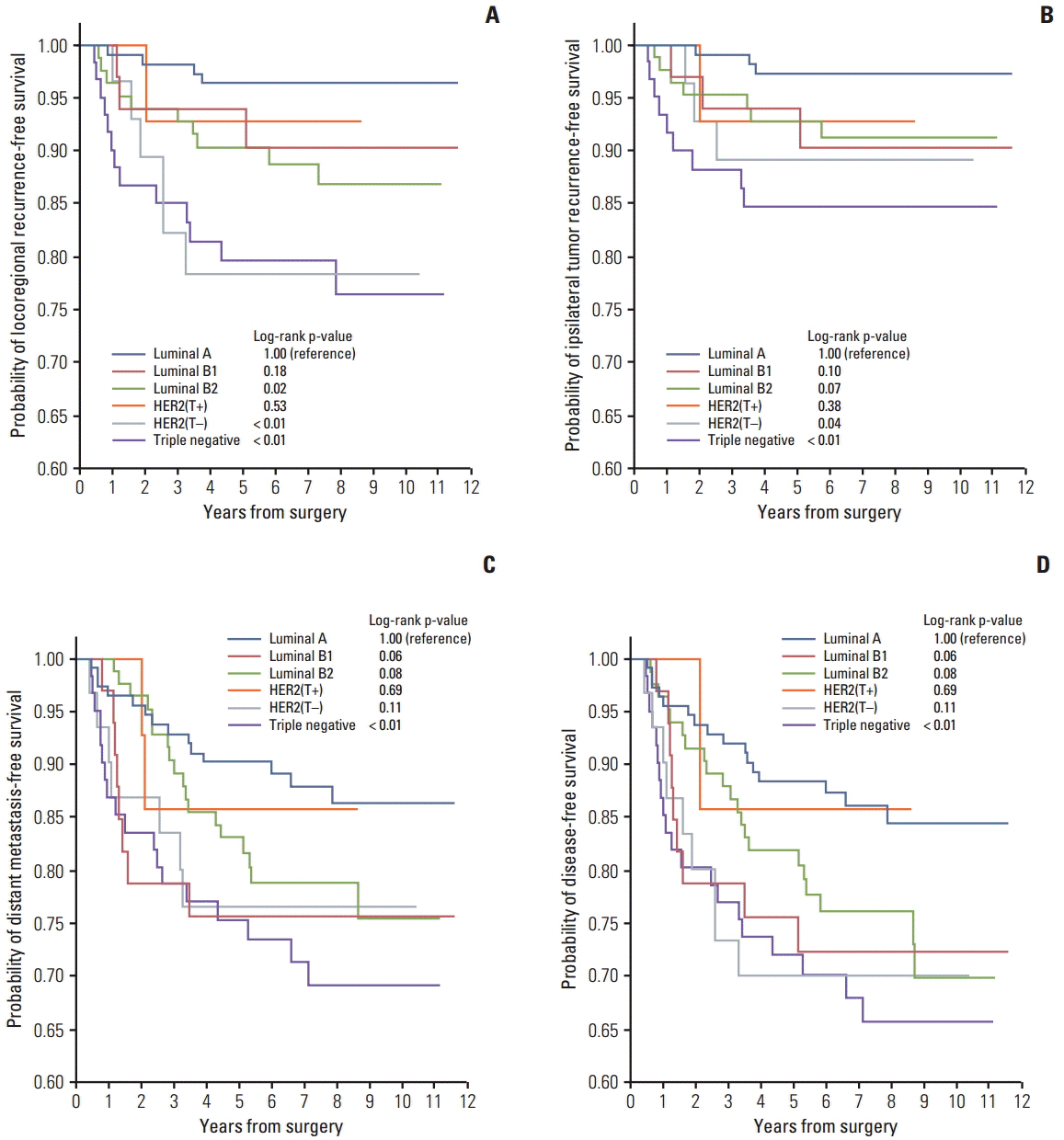
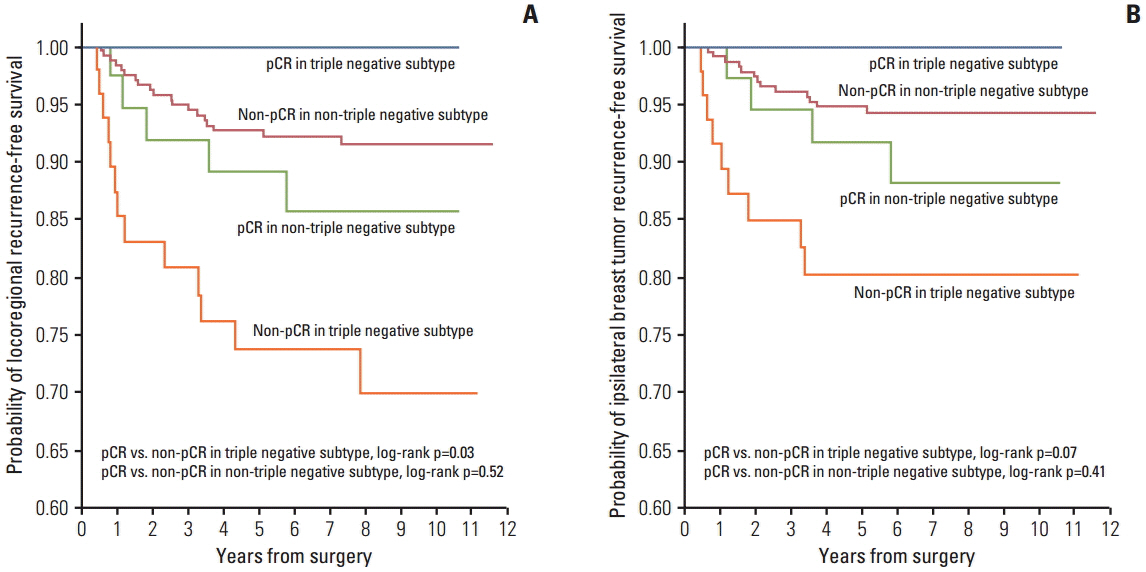
After a median follow-up period of 7.2 years, 26 IBTRs and 37 LRRs occurred. The 5-year LRR-free survival rates were luminal A, 96.4%; B1, 93.9%; B2, 90.3%; HER2(T+), 92.9%; HER2(T-), 78.3%; and TN, 79.6%. The 5-year IBTR-free survival rates were luminal A, 97.2%; B1, 93.9%; B2, 92.8%; HER2(T+), 92.9%; HER2(T-), 89.1%; and TN, 84.6%. In multivariate analysis, HER2(T-) (IBTR: hazard ratio, 4.2; p=0.04 and LRR: hazard ratio, 7.6; p < 0.01) and TN subtypes (IBTR: hazard ratio, 6.9; p=0.01 and LRR: hazard ratio, 8.1; p < 0.01) were associated with higher IBTR and LRR rates. A pathologic complete response (pCR) was found to show correlation with better LRR and a tendency toward improved IBTR controls in TN patients (IBTR, p=0.07; LRR, p=0.03).
CONCLUSION
The TN and HER2(T-) subtypes predict higher rates of IBTR and LRR after NAC and BCT. A pCR is predictive of improved IBTR or LRR in TN subtype.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Schwartz GF, Birchansky CA, Komarnicky LT, Mansfield CM, Cantor RI, Biermann WA, et al. Induction chemotherapy followed by breast conservation for locally advanced carcinoma of the breast. Cancer. 1994; 73:362–9.
Article2. van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001; 19:4224–37.
Article3. Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008; 26:778–85.
Article4. Mieog JS, van der Hage JA, van de Velde CJ. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg. 2007; 94:1189–200.
Article5. Min SY, Lee SJ, Shin KH, Park IH, Jung SY, Lee KS, et al. Locoregional recurrence of breast cancer in patients treated with breast conservation surgery and radiotherapy following neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys. 2011; 81:e697–705.
Article6. Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000; 406:747–52.
Article7. Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003; 100:8418–23.8. Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005; 23:7350–60.
Article9. Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006; 295:2492–502.
Article10. Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008; 26:2373–8.
Article11. Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010; 11:55–65.
Article12. Mamounas EP, Tang G, Fisher B, Paik S, Shak S, Costantino JP, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010; 28:1677–83.
Article13. Lee KS, Ro J, Lee ES, Kang HS, Kim SW, Nam BH, et al. Primary systemic therapy with intermittent weekly paclitaxel plus gemcitabine in patients with stage II and III breast cancer: a phase II trial. Invest New Drugs. 2010; 28:83–90.
Article14. Lee KS, Ro J, Nam BH, Lee ES, Kwon Y, Kwon HS, et al. A randomized phase-III trial of docetaxel/capecitabine versus doxorubicin/cyclophosphamide as primary chemotherapy for patients with stage II/III breast cancer. Breast Cancer Res Treat. 2008; 109:481–9.
Article15. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998; 11:155–68.16. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013; 31:3997–4013.
Article17. Dowsett M, Nielsen TO, A'Hern R, Bartlett J, Coombes RC, Cuzick J, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011; 103:1656–64.
Article18. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–47.19. Stuart-Harris R, Caldas C, Pinder SE, Pharoah P. Proliferation markers and survival in early breast cancer: a systematic review and meta-analysis of 85 studies in 32,825 patients. Breast. 2008; 17:323–34.
Article20. Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001; 98:10869–74.
Article21. Noh JM, Kim J, Cho DY, Choi DH, Park W, Huh SJ. Exome sequencing in a breast cancer family without BRCA mutation. Radiat Oncol J. 2015; 33:149–54.22. Arvold ND, Taghian AG, Niemierko A, Abi Raad RF, Sreedhara M, Nguyen PL, et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol. 2011; 29:3885–91.
Article23. Vargo JA, Beriwal S, Ahrendt GM, Soran A, Johnson RR, McGuire K, et al. Molecular class as a predictor of locoregional and distant recurrence in the neoadjuvant setting for breast cancer. Oncology. 2011; 80:341–9.
Article24. Meyers MO, Klauber-Demore N, Ollila DW, Amos KD, Moore DT, Drobish AA, et al. Impact of breast cancer molecular subtypes on locoregional recurrence in patients treated with neoadjuvant chemotherapy for locally advanced breast cancer. Ann Surg Oncol. 2011; 18:2851–7.
Article25. Caudle AS, Yu TK, Tucker SL, Bedrosian I, Litton JK, Gonzalez-Angulo AM, et al. Local-regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res. 2012; 14:R83.
Article26. Esserman LJ, Berry DA, Cheang MC, Yau C, Perou CM, Carey L, et al. Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012;ACRIN 6657). Breast Cancer Res Treat. 2012; 132:1049–62.27. Esserman LJ, Berry DA, DeMichele A, Carey L, Davis SE, Buxton M, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL--CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012; 30:3242–9.
Article28. Yoo C, Ahn JH, Jung KH, Kim SB, Kim HH, Shin HJ, et al. Impact of immunohistochemistry-based molecular subtype on chemosensitivity and survival in patients with breast cancer following neoadjuvant chemotherapy. J Breast Cancer. 2012; 15:203–10.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Systemic Treatment After Locoregional Recurrence in Breast Cancer: A Review
- Locoregional Recurrence of Breast Conserving Surgery after Preoperative Chemotherapy in Korean Women with Locally Advanced Breast Cancer
- The Impact of Patient Age upon Locoregional and Systemic Failures after Breast Conservation Therapy: Comparison of the Results from the Groups above and below 35 Years
- The Impact of Patient Age upon Locoregional and Systemic Failures after Breast Conservation Therapy: Comparison of the results from the groups above and below 35 years
- Outcome of Surgical Excision for Isolated Locoregional Recurrence of Breast Cancer