J Breast Cancer.
2011 Dec;14(4):289-295. 10.4048/jbc.2011.14.4.289.
Locoregional Recurrence of Breast Conserving Surgery after Preoperative Chemotherapy in Korean Women with Locally Advanced Breast Cancer
- Affiliations
-
- 1Center for Breast Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Korea. eslee@nee.re.kr
- KMID: 2175722
- DOI: http://doi.org/10.4048/jbc.2011.14.4.289
Abstract
- PURPOSE
Preoperative chemotherapy has been used to increase the rate of breast conserving surgery (BCS) in Caucasian women. However, whether it would also increase the rate of BCS in Korean women has not been verified. The aim of this study was to determine the effectiveness of preoperative chemotherapy to make BCS possible in Korean women who have locally advanced cancer without any increase of locoregional recurrence according to operation methods (BCS vs. mastectomy).
METHODS
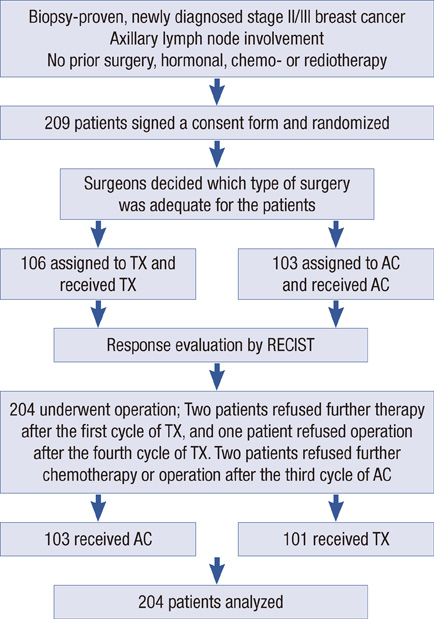
From August 2002 to April 2005, 205 patients with stage II or III breast cancer were enrolled in a phase III randomized trial of preoperative chemotherapy. Surgeons decided on the type of surgery (mastectomy or BCS) at initial diagnosis. By randomization, patients received four cycles of either docetaxel/capecitabine or doxorubicin/cyclophosphamide followed by surgery and crossover to the other treatment as postoperative chemotherapy.
RESULTS
The mean tumor size was 3.29 cm and the mean breast volume was 489 cc at diagnosis. After preoperative chemotherapy, clinical response was shown in 76.0% of the patients. Of the 71 patients planned for a mastectomy at initial diagnosis, 27 patients underwent BCS (38.0%). Clinical T stage after preoperative chemotherapy, pathologic T size and lymphatic invasion were correlated with conversion to BCS. In multivariate analysis, only lymphatic invasion showed statistical significance. Locoregional disease-free survival did not statistically differ between the two operation methods for the patients who were planned for a mastectomy at the initial exam.
CONCLUSION
This study showed that preoperative chemotherapy also increased the rate of BCS, while avoiding any increase of locoregional recurrence in Korean women with locally advanced breast cancer.
MeSH Terms
Figure
Cited by 1 articles
-
Impact of Molecular Subtype Conversion of Breast Cancers after Neoadjuvant Chemotherapy on Clinical Outcome
Siew Kuan Lim, Moo Hyun Lee, In Hae Park, Ji Young You, Byung-Ho Nam, Byeong Nam Kim, Jungsil Ro, Keun Seok Lee, So-Youn Jung, Young Mee Kwon, Eun Sook Lee
Cancer Res Treat. 2016;48(1):133-141. doi: 10.4143/crt.2014.262.
Reference
-
1. Wolff AC, Davidson NE. Preoperative therapy in breast cancer: lessons from the treatment of locally advanced disease. Oncologist. 2002. 7:239–245.
Article2. Buzdar AU. Preoperative chemotherapy treatment of breast cancer: a review. Cancer. 2007. 110:2394–2407.
Article3. Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997. 15:2483–2493.
Article4. Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998. 16:2672–2685.
Article5. Ahn SH, Yoo KY. Korean Breast Cancer Society. Chronological changes of clinical characteristics in 31,115 new breast cancer patients among Koreans during 1996-2004. Breast Cancer Res Treat. 2006. 99:209–214.
Article6. Lee KS, Ro J, Nam BH, Lee ES, Kwon Y, Kwon HS, et al. A randomized phase-III trial of docetaxel/capecitabine versus doxorubicin/cyclophosphamide as primary chemotherapy for patients with stage II/III breast cancer. Breast Cancer Res Treat. 2008. 109:481–489.
Article7. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. European Organization for Research and Treatment of Cancer. National Cancer Institute of the United States. National Cancer Institute of Canada. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000. 92:205–216.
Article8. Inoue T, Yutani K, Taguchi T, Tamaki Y, Shiba E, Noguchi S. Preoperative evaluation of prognosis in breast cancer patients by [(18)F]2-Deoxy-2-fluoro-D-glucose-positron emission tomography. J Cancer Res Clin Oncol. 2004. 130:273–278.
Article9. Wahl RL, Siegel BA, Coleman RE, Gatsonis CG. PET Study Group. Prospective multicenter study of axillary nodal staging by positron emission tomography in breast cancer: a report of the staging breast cancer with PET Study Group. J Clin Oncol. 2004. 22:277–285.
Article10. Zornoza G, Garcia-Velloso MJ, Sola J, Regueira FM, Pina L, Beorlegui C. 18F-FDG PET complemented with sentinel lymph node biopsy in the detection of axillary involvement in breast cancer. Eur J Surg Oncol. 2004. 30:15–19.
Article11. Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001. (30):96–102.
Article12. Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006. 24:2019–2027.
Article13. Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003. 21:4165–4174.
Article14. Mauriac L, MacGrogan G, Avril A, Durand M, Floquet A, Debled M, et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up. Institut Bergonié Bordeaux Groupe Sein (IBBGS). Ann Oncol. 1999. 10:47–52.
Article15. Scholl SM, Fourquet A, Asselain B, Pierga JY, Vilcoq JR, Durand JC, et al. Neoadjuvant versus adjuvant chemotherapy in premenopausal patients with tumours considered too large for breast conserving surgery: preliminary results of a randomised trial: S6. Eur J Cancer. 1994. 30A:645–652.
Article16. van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001. 19:4224–4237.
Article17. Semiglazov VF, Topuzov EE, Bavli JL, Moiseyenko VM, Ivanova OA, Seleznev IK, et al. Primary (neoadjuvant) chemotherapy and radiotherapy compared with primary radiotherapy alone in stage IIb-IIIa breast cancer. Ann Oncol. 1994. 5:591–595.
Article18. Powles TJ, Hickish TF, Makris A, Ashley SE, O'Brien ME, Tidy VA, et al. Randomized trial of chemoendocrine therapy started before or after surgery for treatment of primary breast cancer. J Clin Oncol. 1995. 13:547–552.
Article19. Gianni L, Baselga J, Eiermann W, Porta VG, Semiglazov V, Garcia-Conde J, et al. First report of the European Cooperative Trial in operable breast cancer (ECTO): effects of primary systemic therapy PST on local-regional disease. 2002. 21:In : 2002 American Society of Clinical Oncology Annual Meeting; Abstract #132.20. Viswambharan JK, Kadambari D, Iyengar KR, Srinivasan K. Feasibility of breast conservation surgery in locally advanced breast cancer downstaged by neoadjuvant chemotherapy: a study in mastectomy specimens using simulation lumpectomy. Indian J Cancer. 2005. 42:30–34.
Article21. Zhou B, Yang DQ, Qiao XM, Tong FZ, Cao YM, Liu P, et al. Feasibility of breast conservation surgery after neoadjuvant chemotherapy for breast cancer. Zhonghua Yi Xue Za Zhi. 2005. 85:769–772.22. Sadetzki S, Oberman B, Zipple D, Kaufman B, Rizel S, Novikov I, et al. Breast conservation after neoadjuvant chemotherapy. Ann Surg Oncol. 2005. 12:480–487.
Article23. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002. 347:1233–1241.
Article24. van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000. 92:1143–1150.
Article25. Jacobson JA, Danforth DN, Cowan KH, d'Angelo T, Steinberg SM, Pierce L, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med. 1995. 332:907–911.
Article26. Veronesi U, Bonadonna G, Zurrida S, Galimberti V, Greco M, Brambilla C, et al. Conservation surgery after primary chemotherapy in large carcinomas of the breast. Ann Surg. 1995. 222:612–618.
Article27. McIntosh SA, Ogston KN, Payne S, Miller ID, Sarkar TK, Hutcheon AW, et al. Local recurrence in patients with large and locally advanced breast cancer treated with primary chemotherapy. Am J Surg. 2003. 185:525–531.
Article28. Touboul E, Lefranc JP, Blondon J, Buffat L, Deniaud E, Belkacémi Y, et al. Primary chemotherapy and preoperative irradiation for patients with stage II larger than 3 cm or locally advanced non-inflammatory breast cancer. Radiother Oncol. 1997. 42:219–229.
Article29. Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008. 26:778–785.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation between Tumor Response to Neoadjuvant Chemotherapy and Patient Outcome in Breast Cancer
- Correlation between Tumor Response to Neoadjuvant Chemotherapy and Patient Outcome in Breast Cancer
- Radiotherapy for Breast Cancer
- Systemic Treatment After Locoregional Recurrence in Breast Cancer: A Review
- Comparison of Psychiatric Symptoms between Total Mastectomy and Breast Conserving Surgery in Breast Cancer Patients